Abstract
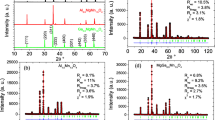
Manganese-gallium samples with cation ratios Mn:Ga = 1:2, 1.5:1.5, and 2:1 are synthesized by coprecipitation with subsequent annealing in air in a temperature range 600–1200 °C. Powder XRD, TEM, and BET methods are used to study the physicochemical characteristics of the samples. It is found that in the air at the annealing temperature of 600 °C finely dispersed low-temperature Mn3–xGaxO4 spinels primarily form in all series, but in the whole temperature range (600–1200 °C) the system is multiphase. Annealing at 800–1200 °C leads to an increase in the concentration of simple oxides (β-Mn3O4 and β-Ga2O3). Only simple α-Mn2O3 and β-Ga2O3 oxides exist in a Mn:Ga = 2:1 series at 800 °C. In the sample with a cation ratio Mn:Ga = 1.5:1.5 annealed in air at 1000 °C, the formation of a superstructure based on the spinel structure is found.
Similar content being viewed by others
References
S. I. Galanov, A. I. Galanov, M. Y. Smirnov, O. I. Sidorova, and L. N. Kurina. Izv. Tomsk. Politekh. Inst., 2005, 308,126.
P. G. Tsyrul'nikov. Ross. Khim. Zh., 2007, 51,133.
S. I. Galanov, O. I. Sidorova, E. A. Litvak, and K. A. Kosyreva. Izv. Tomsk. Politekh. Inst., 2012, 320,124.
M. Tepluchin, M. Casapu, A. Boubnov, H. Lichtenberg, D. Wang, S. Kureti, and J.-D. Grunwaldt. ChemCatChem, 2014, 6, 1763–1773.
O. A. Bulavchenko, T. N. Afonasenko, P. G. Tsyrul'nikov, and S. V. Tsybulya. Appl. Catal., A, 2013, 459,73.
S. V. Tsybulya, G. N. Kryukova, T. A. Kriger, and P. G. Tsyrul'nikov. Kinet. Katal., 2003, 44,318.
O. A. Bulavchenko, S. V. Tsybulya, S. V. Cherepanova, T. N. Afonasenko, and P. G. Tsyrul'nikov. J. Struct. Chem., 2009, 50,497.
O. A. Bulavchenko. Structural Aspects of Activation of Oxide Aluminum-Cobalt and Aluminum-Manganese Catalysts. Diss. … Cand. of Chem. Sc. [in Russian]. Novosibirsk: Institute of Catalysis, Siberian Branch of the RAS, 2010.
O. A. Bulavchenko, S. V. Tsybulya, P. G. Tsyrul'nikov, T. N. Afonasenko, S. V. Cherepanova, and E. Yu. Gerasimov. J. Struct. Chem., 2010, 51,518.
I. Levin and D. Brandon. J. Am. Ceram. Soc., 1998, 81, 1995.
S. V. Tsybulya and G. N. Kryukova. Phys. Rev. B, 2008, 77, 0241121.
H. Y. Playford, A. C. Hannon, E. R. Barney, and R. I. Walton. Chem. -Eur. J., 2013, 19, 2803.
O. S. Venediktova, O. A. Bulavchenko, T. N. Afonasenko, P. G. Tsyrul'nikov, Z. S. Vinokurov, Y. A. Chesalov, and S. V. Tsybulya. J. Alloys Comp., 2017, 725,496.
O. S. Venediktova, O. A. Bulavchenko, P. G. Tsyrul'nikov, T. N. Afonasenko, Z. S. Vinokurov, and S. V. Tsybulya. J. Struct. Chem., 2018, 59,370.
ICDD PDF-4+. Dr. Soorya Kabekkodu, Editor. Newtown Square, PA, USA, 2012.
S. V. Tsybulya, S. V. Cherepanova, and L.P. Soloviyova. Zh. Struk. Khim., 1996, 37,332.
P. Scherrer. Bestimmung der Grösse und der inneren Struktur von Kolloidteilchen mittels Röntgenstrahlen. Nachr. Ges. Wiss. Goettingen, Math.-Phys. Kl., 1918, 1918, 98–100.
M. Wojdyr. J. Appl. Crystallogr., 2010, 43, 1126.
P. G. Casado and I. Rasines. Z. Kristallogr., 1982, 160,33.
M. Lensen, A. Michel, and C. R. Hebd. Seances Acad. Sci., 1958, 246, 1997.
O. Schmitz-DuMont, H. Brokopf, and K. Burkhardt. Z. Anorg. Allg. Chem., 1958, 295,7.
J. Böhm. Angew. Chem., 1940, 63,131.
C. O. Arean, A. L. Bellan, M. P. Mentruit, M. R. Delgado, and G. T. Palomino. Microporous Mesoporous Mater., 2000, 40,35.
K. Pohl. Naturwiss, 1968, 55,82.
M. Zinkevich, F. M. Morales, H. Nitsche, M. Ahrens, M. Rühle, and F. Aldinger. Z. Metallkd., 2004, 95,756.
H. Y. Playford, A. C. Hannon, E. R. Barney, and R. I. Walton. Chem. Eur. J., 2013, 19, 2803.
H. Y. Playford, A. C. Hannon, M. G. Tucker, D. M. Dawson, S. E. Ashbrook, R. J. Kastiban, J. Sloan, and R. I. Walton. J. Phys. Chem. C, 2014, 118, 16188.
O. Nikulina, D. Yatsenko, O. Bulavchenko, G. Zenkovets, and S. Tsybulya. Z. Kristallogr., 2016, 231,261.
V. F. Balakirev, V. P. Barkhatov, Yu. V. Golikov, and S. G. Maizel. Manganites: Equilibrium and Unstable States [in Russian]. Ekaterinburg: Ural Branch of the RAS, 2000.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © 2018 O. S. Venediktova, O. A. Bulavchenko, T. N. Afonasenko, P. G. Tsysul′nikov, E. Yu. Gerasimov, S. V. Tsybulya.
Translated from Zhurnal Strukturnoi Khimii, Vol. 59, No. 7, pp. 1689–1696, September-October, 2018.
Rights and permissions
About this article
Cite this article
Venediktova, O.S., Bulavchenko, O.A., Afonasenko, T.N. et al. Phase Transformations in the Mn–Ga–O System Depending on the Preparation Conditions. J Struct Chem 59, 1631–1638 (2018). https://doi.org/10.1134/S0022476618070156
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022476618070156