Abstract
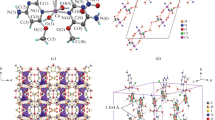
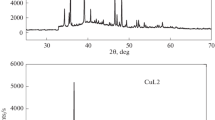
(C6H18N3)4[CuCl5]2[CuCl4]3·1.42H2O is prepared and characterized by various physicochemical techniques. The single crystal X-ray diffraction structural analysis reveals that the title compound belongs to the orthorhombic system with the space group Cmca. Its unit cell dimensions are: a = 24.286(2) Å, b = 14.3082(14) Å, c = 16.6160(16) Å, Z = 4, V = 5773.8(10) Å3. Its crystal structure is determined and refined down to R = 0.024 and wR(F2) = 0.059. The structure contains three crystallographically independent Cu2+ ions coordinated to chlorine anions in various fashions. Cu1 is five-coordinated in a distorted square pyramidal fashion, while Cu2 and Cu3 are four-coordinated in square planar and distorted tetrahedral fashions, respectively. The entities are interconnected by means of the hydrogen bonding [O(W)–H…Cl, N–H…Cl, C–H…Cl and C–H…O(W)], forming a three-dimensional network. Intermolecular interactions are investigated by Hirshfeld surfaces and the contacts of the eight different chloride atoms are notably compared. The vibrational absorption bands are identified by infrared spectroscopy. The optical study is performed by UV-vis absorption.
Similar content being viewed by others
References
J. D. Martin and B. R. Leafblad. Angew. Chem., 1998, 37, 3318–3320.
C. R. Rice, S. Onions, N. Vidal, J. D. Wallis, M.-C. Senna, M. Pilkington, and H. Stoeckli-Evans. Eur. J. Inorg. Chem., 2002, 8, 1985–1997.
R. Bhattacharya, M. S. Ray, R. Dey, L. Righi, G. Bocelli, and A. Ghosh. Polyhedron, 2002, 21, 2561–2565.
A. Weselucha-Birczynska and C. Paluszkiewicz. J. Mol. Struct., 2002, 614, 339–343.
S. F. Haddad, M. A. AlDamen, and R. D. Willet. Inorg. Chim. Acta, 2006, 359, 424–432.
A. R. Parent, C. P. Landee, and M. M. Turnbull. Inorg. Chim. Acta, 2007, 360, 1943–1953.
F. A. Cotton, L. M. Daniels, and P. I. Huang. Inorg. Chem., 2001, 40, 3576–3578.
M. Czugler, L. Kótai, B. Sreedhar, A. Rockenbauer, I. Gács, and S. Holly. Eur. J. Inorg. Chem., 2002, 12, 3298–3304.
N. Lah and R. Clérac. Polyhedron, 2009, 28, 2466–2472.
B. J. Prince, M. M. Turnbull, and R. D. Willett. J. Coord. Chem., 2003, 56, 441–452.
S. N. Herringer, M. M. Turnbull, C. P. Landee, and J. L. Wikaira. J. Coord. Chem., 2009, 62, 863–875.
M. Zdanowska-Fraczek, K. Holderna-Natkaniec, Z. J. Fraczek, and R. Jakubas. Solid State Ion, 2009, 180, 9–12.
I. Chaabane, F. Hlel, and K. Guidara. J. Alloys Compd., 2008, 461, 495–500.
K. Sakai, M. Takemura, and Y. Kawabe. J. Lumin., 2010, 130, 2505–2507.
K. Pradeesh, G. Sharachandar Yadav, M. Singh, and G. Vijaya Prakash. J. Mater. Chem. Phys., 2010, 124, 44–47.
M. Bujak and J. Zaleski. Cryst. Eng., 2001, 4, 241–252.
P. Gomez-Romero. Adv. Mater., 2001, 13, 163–174.
John R. J. Sorenson. J. Med. Chem., 1976, 19, 135–148.
P. M. May and D. R. Williams. Met. Ions Biol. Syst., Helmut Sigel: New York, USA, 1981, 12, 283–317.
P. S. Subramanian and D. Srinivas. Polyhedron, 1996, 15, 985–989.
J. Sertucha, A. Luque, F. Lloret, and P. Román. Polyhedron, 1998, 17, 3875–3880.
V. Fernandez, M. Moran, M. T. Gutiérrez-Rios, C. Foces-Foces, and F. H. Cano. Inorg. Chim. Acta, 1987, 128, 239–243.
P. Román, J. Sertucha, A. Luque, L. Lezama, and T. Rojo. Polyhedron, 1996, 15, 1253–1262.
K. M. Guckian, B. A. Schweitzer, R. X. Ren, C. J. Sheils, D. C. Tahmassebi, and E. T. Kool. J. Am. Chem. Soc., 2000, 122, 2213–2222.
Bruker Apex2, Advanced X-ray Solutions Bruker AXS Inc. Madison, Wisconsin, USA, 2009.
G. M. Sheldrick. Acta Crystallogr., 2008, A64, 112–122.
Bruker Advanced X-ray Solutions, SHELXTL (Version 6.14), Bruker AXS Inc. Madison, Wisconsin, USA, 2003.
G. M. Sheldrick. SHELXL2013, University of Göttingen, Germany, 2013.
C. B. Hübschle, G. M. Sheldrick, and B. Dittrich. J. Appl. Crystallogr., 2011, 44, 1281–1284.
K. Brandenburg. Diamond Version 2.0 Impact GbR, Bonn., Germany, 1998.
L. Yang, D. R. Powell, and R. P. Houser. Dalton Trans., 2007, 955–964.
A. Kessentini, M. Belhouchet, Y. Abid, C. Minot, and T. Mhiri. Spectrochim. Acta A, 2014, 122, 476–481.
D.-H. He, Y.-Y. Di, Z.-C. Tan, F.-F. Yi, W.-Y. Dan, and Y.-P. Liu. Sol. Energy Mater. Sol. Cells, 2011, 95, 2897–2906.
E. P. Aldrich, K. A. Bussey, J. R. Connell, E. F. Reinhart, K. D. Oshin, B. Q. Mercado, and A. G. Oliver. Acta Crystallogr., 2016, E72, 40–43.
A. Kessentini, M. Belhouchet, J. J. Suñol, Y. Abid, and T. Mhiri. J. Lumin., 2014, 149, 341–347.
G. R. Willey, M. Ravindran, and M. G. B. Drew. Inorg. Chim. Acta, 1991, 188, 159–162.
A. W. Addison, T. Nageswara Rao, J. Recdijk, J. van Rijn, and G. C. Verschoor. J. Chem. Soc., Dalton Trans., 1984, 1349–1356.
D. Cremer. J. Am. Chem. Soc., 1975, 97, 1354–1358.
M. A. Spackman and D. Jayatilaka. CrystEngComm, 2009, 11, 19–32.
B. Guillot, E. Enrique, L. Huder, and C. Jelsch. Acta Crystallogr., 2014, A70, C279.
C. Jelsch, S. Soudani, and C. Ben Nasr. IUCrJ, 2015, 2, 327–340.
S. Gunasekaran and B. Anita. Indian J. Pure Appl. Phys., 2008, 46, 833–838.
J. Orive, E. S. Larrea, R. F. De Luis, M. Iglesias, J. L. Mesa, T. Rojo, and M. I. Arriortua. Dalton Trans., 2013, 42, 4500–4512.
P. F. Raphael, E. Manoj, and M. R. P. Kurup. Polyhedron, 2007, 26, 818–828.
B. F. Hathaway. In Comprehensive Coordination Chemistry /Ed. G. Wilkinson. Pergamon, Oxford, 1st edn., 1987, 5, 533–774.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © 2018 Maroua El Glaoui, Maher El Glaoui, C. Jelsch, C. Ben Nasr.
The text was submitted by the authors in English. Zhurnal Strukturnoi Khimii, Vol. 59, No. 7, pp. 1669–1677, September-October, 2018.
Rights and permissions
About this article
Cite this article
El Glaoui, M., El Glaoui, M., Jelsch, C. et al. Hirshfeld Surface Analysis, Crystal Structure and Spectroscopic Studies of a New Cu(II) Halocuprate Salt with Protonated N-Amino-Ethyl-Piperazine. J Struct Chem 59, 1610–1618 (2018). https://doi.org/10.1134/S0022476618070120
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022476618070120