Abstract
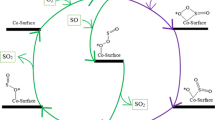
It is of high importance to finding efficient catalysts for oxidation of nitrogen (N2) and fluorine monoxide (FO) molecules. In this study, Ge–B36N36 and Sn–BNNT are formed and the surfaces of Ge–B36N36 and Sn–BNNT via the O2 molecule are activated. Oxidation of N2 and FO on the surfaces of O2–Ge–B36N36 and O2–Sn–BNNT via Langmuir–Hinshelwood (LH) and Eley–Rideal (ER) mechanisms are investigated. The results show that O2–Ge–B36N36 and O2–Sn–BNNT can oxidize the N2 and FO molecules via two-step reactions, respectively. Results show that N2 and FO oxidation on the O2–Ge–B36N36 and O2–Sn–BNNT surfaces via the LH mechanism has a lower energy barrier than that of the ER mechanism. Finally, O2–Ge–B36N36 and O2–Sn–BNNT are acceptable catalysts with a high performance for the oxidation of N2 and FO molecules, respectively.
Similar content being viewed by others
References
C. Zhang, B. Yoon, and U. Landman. J. Am. Chem. Soc., 2007, 129, 2228/2229.
J. Zhang, H. Jin, M. B. Sullivan, F. C. H. Lim, and P. Wu. Phys. Chem. Chem. Phys., 2009, 11, 1441–1446.
N. Lopez, T. Janssens, B. Clausen, Y. Xu, M. Mavrikakis, T. Bligaard, and J. K. Nørskov. J. Catal., 2004, 223, 232–235.
H.–Y. Su, M.–M. Yang, X.–H. Bao, and W.–X. Li. J. Phys. Chem. C, 2008, 112, 17303–17310.
S. Piccinin and M. Stamatakis. ACS Catal., 2014, 4, 2143–2152.
M. S. Chen, Y. Cai, Z. Yan, K. K. Gath, S. Axnanda, and D. W. Goodman. Surf. Sci., 2007, 601, 5326–5331.
A. K. Geim and K. S. Novoselov. Nat. Mater., 2007, 6, 183–191.
K. S. Novoselov, A. K. Geim, S. Morozov, D. Jiang, Y. Zhang, S. A. Dubonos, I. Grigorieva, and A. Firsov. Science, 2004, 306, 666–669.
Y. Huang, J. Liang, and Y. Chen. Small, 2012, 8, 1805–1834.
M. D. Stoller, S. Park, Y. Zhu, J. An, and R. S. Ruoff. Nano Lett., 2008, 8, 3498–3502.
H. Lee, J. Ihm, M. L. Cohen, and S. G. Louie. Nano Lett., 2010, 10, 793–798.
G. K. Dimitrakakis, E. Tylianakis, and G. E. Froudakis. Nano Lett., 2008, 8, 3166–3170.
G. Eda, G. Fanchini, and M. Chhowalla. Nat. Nanotechnol., 2008, 3, 270–274.
W. Choi, I. Lahiri, R. Seelaboyina, and Y. S. Kang. Crit. Rev. Solid State, 2010, 35, 52–71.
C. O. Girit, J. C. Meyer, R. Erni, M. D. Rossell, C. Kisielowski, L. Yang, C. H. Park, M. F. Crommie, M. L. Cohen, S. G. Louie, and A. Zettl. Science, 2009, 323, 1705–1708.
A. C. Neto, F. Guinea, N. Peres, K. S. Novoselov, and A. K. Geim. Rev. Mod. Phys., 2009, 81, 109.
B. Guo, L. Fang, B. Zhang, and J. R. Gong. Insciences J., 2011, 80–89.
Y.–H. Lu, M. Zhou, C. Zhang, and Y.–P. Feng. J. Phys. Chem. C, 2009, 113, 20156–20160.
F. Li, J. Zhao, and Z. Chen. J. Phys. Chem. C, 2012, 116, 2507–2514.
L. Wang, Q. Luo, W. Zhang, and J. Yang. Int. J. Hydrogen Energy, 2014, 39, 20190–20196.
G. H. Lee. Ceram. Int., 2013, 39, 7989–7993.
S. M. Vesecky, J. Paul, and D. W. Goodman. J. Phys. Chem., 1996, 100, 15242–15246.
M. A. Farrokhzad and T. I. Khan. Mater. Sci. Eng., 2014, 60, 012011–012014.
W. H. Moon, M. S. Son, and H. J. Hwang. Appl. Sur. Sci., 2007, 253, 7078–7081.
P. W. Fowler, T. Heine, D. Mitchell, R. Schmid, and G. Seifert. J. Chem. SOC., Faraday Trans., 1996, 12, 2197–2201.
H. Si, G. Lian, A. Wang, D. Cui, M. Zhao, Q. Wang, and C. Wong. Nano Lett., 2015, 15, 8122–8128.
T. Oku and M. Kuno. Diamond Relat. Mater., 2003, 12, 840–845.
T. Oku, I. Narita, A. Nishiwaki. Mater Manuf Process, 2004, 19, 1215–1239.
L. Han and P. Krstic. Nanotechnology, 2017, 28, 701–706.
T. Wehling, K. Novoselov, S. Morozov, E. Vdovin, M. Katsnelson, A. Geim, and A. Lichtenstein. Nano Lett., 2008, 8, 173–177.
M. Zhao and D. G. Truhlar. Theor. Chem. Acc., 2008, 120, 215–241.
M. D. Esrafili and R. Nurazar. Comput. Mater. Sci., 2014, 92, 172–177.
Y. Li, Z. Zhou, G. Yu, W. Chen, and Z. Chen. J. Phys. Chem. C, 2010, 114, 6250–6254.
C. Huang, X. Ye, C. Chen, S. Lin, and D. Xie. Comput. Theor. Chem., 2013, 1011, 5–10.
S. Wannakao, T. Nongnual, P. Khongpracha, T. Maihom, and J. Limtrakul. J. Phys. Chem. C, 2012, 116, 16992–16998.
P. Zhao, Y. Su, Y. Zhang, S.–J. Li, and G. Chen. Chem. Phys. Lett., 2011, 515, 159–162.
E. H. Song, J. M. Yan, J. S. Lian, and Q. Jiang. J. Phys. Chem. C, 2012, 116, 20342–20348.
M. D. Esrafili, P. Nematollahi, and H. Abdollahpour. Appl. Surf. Sci., 2016, 378, 418–425.
M. D. Esrafili and N. Saeidi. Phys. E, 2015, 74, 382–387.
M. D. Esrafili, P. Nematollahi, and R. Nurazar. Superlattices Microstruct., 2016, 92, 60–67.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © 2018 S. A. Pourabadeh, B. Nasrollahzadeh, R. Razavi, A. Bozorgian, M. Najafi.
The text was submitted by the authors in English. Zhurnal Strukturnoi Khimii, Vol. 59, No. 6, pp. 1536–1453, July-August, 2018.
Rights and permissions
About this article
Cite this article
Pourabadeh, A., Nasrollahzadeh, B., Razavi, R. et al. Oxidation of FO and N2 Molecules on the Surfaces of Metal-Adopted Boron Nitride Nanostructures as Efficient Catalysts. J Struct Chem 59, 1484–1491 (2018). https://doi.org/10.1134/S0022476618060355
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022476618060355