Abstract
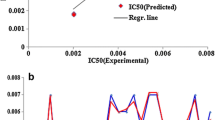
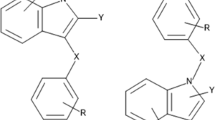
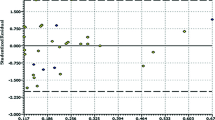
The random sampling analysis on molecular surface (RASMS) method is applied to study quantitative structure activity relationships (QSARs) modeled by multiple linear regression for dihydroalkoxybenzyloxopyrimidines (DABO) derivatives as HIV-1 reverse transcriptase non-nucleoside inhibitors. The correlation coefficients (R2cum) and cross-validation correlation coefficients (Q2CV) obtained by the models are 0.852 and 0.755 for 74 DABO derivatives. Satisfactory results show that information about the biological activity can be systemically expressed by the RASMS method that can be a useful structural expression methodology for the study of QSAR of the DABO derivatives.
Similar content being viewed by others
References
A. S. Fauci, Science, 239, 617 (1998).
E. D. Clercq, J. Med. Chem., 38, 2491 (1995).
C. Ahgren, K. Backro, F. W. Bell, A. S. Cantrell, M. Clemens, J. M. Colacino, J. B. Deeter, J. A. Engelhardt, M. Hogberg, S. R. Jaskunas, and N. G. Johansson, Antimicrob. Agents Chemother., 39, 1329 (1995).
R. Pauwels, K. Andries, J. Desmyter, D. Schols, M. J. Kukla, H. J. Breslin, A. Raeymaeckers, J. V. Gelder, R. Woestenborghs, and J. Heykants, Nature, 343, 470 (1990).
M. Baba, H. Tanakas, E. D. Clercq, R. Pauwels, J. Balzarini, D. Schols, H. Nakashima, C. F. Perno, R. T. Walker, and T. Miyasaka, Biochem. Biophys. Res. Commun., 165, 1375 (1989).
J. P. Kleim, R. Bender, U. M. Billhardt, C. Meichsner, G. Riess, M. Rosner, I. Winkler, and A. Paessens, Antimicrob. Agents Chemother., 37, 1659 (1993).
R. Pauwels, K. Andries, Z. Debyser, P. V. Daele, D. Schols, A. M. Vandamme, C. G. M. Janssen, J. Anne, G. J. Desmyter, J. Heykants, M. A. C. Janssen, E. D. Clercq, and P. A. J. Janssen, Proc. Natl. Acad. Sci. USA, 90, 1711 (1993).
J. Balzarini, M. J. Perez, A. S. Felix, D. Schols, D. C. F. Perno, A. Vandamme, M. J. Camarasa, and E. D. Clercq, Proc. Natl. Acad. Sci. USA, 89, 4392 (1992).
D. L. Romero, M. Busso, C. K. Tan, F. Reusser, J. R. Palmer, S. M. Poppe, P. F. Aristoff, K. M. Downey, A. G. L. Resnick, and W. G. Tarpley, Proc. Nat. Acad. Sci., 88, 8806 (1991).
V. J. Merluzzi, K. D. Hargrave, M. Labadia, K. Grozinger, M. Skoog, J. C. Wu, C. K. Shih, K. Eckner, S. Hattox, and J. Adams, Science, 250, 1411 (1990).
S. Razieh, F. Afshin, and M. Behzad, J. Mol. Graphics Modell., 28, 146 (2009).
S. G. Sarafianos, K. Das, S. H. Hughes, and E. Arnold, Curr. Opin. Struct. Biol., 14, 716 (2004).
K. Das, P. J. Lewi, S. H. Hughes, and E. Arnold, Prog. Biophys. Mol. Biol., 88, 209 (2005).
Y. H. Liang and F. E. Chen, Eur. J. Med. Chem., 44, 625 (2009).
B. Hemmateenejad, R. Miri, M. Akhond, and M. Shamsipur, Chemom. Intell. Lab. Syst., 64, 91 (2002).
B. Hemmateenejad, R. Miri, M. Akhond, and M. Shamsipur, Arch. Pharm. Med. Chem., 10, 472 (2002).
C. Hansch, D. Hoekman, and H. Gao, Chem. Rev., 96, 1045 (1996).
A. Fassihi and R. Sabet, Int. J. Mol. Sci., 9, 1876 (2008).
A. Fassihi, D. Abedi, L. Saghaie, R. Sabet, H. Fazeli, G. H. Bostaki, O. Deilami, and H. Sadinpour, Eur. J. Med. Chem., 44, 2145 (2009).
A. Nandy, S. Kar, and K. Roy, SAR QSAR Environ. Res., 44, 1062 (2013).
B. Vyas, O. Silakari, B. M. Singh, and B. Singh, SAR QSAR Environ. Res., 24, 733 (2013).
R. K. Kar, P. Suryadevara, B. R. Sahoo, G. C. Sahoo, M. R. Dikhit, and P. Das, SAR QSAR Environ. Res., 24, 215 (2013).
B. Hemmateenejad and M. Sanchooli, J. Chemom., 21, 96 (2007).
S. A. Kustrin, I. G. Tucker, M. Zecevic, and L. J. Ziva-novic, Anal. Chem. Acta, 418, 181 (2000).
J. B. Tong, T. Che, S. L. Liu, Y. F. Li, P. Wang, X. M. Xu, and Y. Chen, Arch. Pharm. Chem. Life Sci., 344, 719 (2011).
M. Hahn, J. Med. Chem., 38, 2080 (1995).
A. Bondi, J. Phys. Chem., 68, 441 (1964).
J. F. Pei, Q. Wang, J. J. Zhou, and L. H. Lai, Proteins, 57, 651 (2004).
P. Zhou, J. B. Tong, F. F. Tian, and Z. L. Li, Chin. Sci. Bull., 51, 1824 (2006).
L. N. Xu, G. Z. Liang, H. Mei, H. L. Zheng, P. Zhou, J. Wang, and Z. L. Li, Asian J. Ecotoxicol., 1, 72 (2008).
J. B. Tong, T. Che, Y. F. Li, P. Wang, X. M. Xu, and Y. Chen, SAR QSAR Environ. Res., 22, 611 (2011).
J. B. Tong, Y. Chen, S. L. Liu, and X. M. Xu, Med. Chem. Res., 12, 4946 (2013).
J. B. Tong, Y. Chen, S. L. Liu, T. Che, and X. M. Xu, J. Chemom., 26, 549 (2012).
V. D. Sofie and B. Patrick, J. Mol. Struct: THEOCHEM, 943, 83 (2010).
J. B. Tong, S. L. Liu, P. Zhou, B. L. Wu, and Z. L. Li, J. Theor. Biol., 253, 90 (2008).
A. B. Monique, C. R. Rodrigues, J. J. V. Cirino, R. B. Alencastro, H. C. Castro, and M. G. Albuquerque, J. Chem. Inf. Model., 48, 1706 (2008).
K. Roy and I. Mitra, Comb. Chem. High Throughput Screening, 14, 450 (2011).
K. Roy, I. Mitra, S. Kar, P. K. Ojha, R. N. Das, and H. Kabir, J. Chem. Inf. Modell., 52, 396 (2012).
K. Roy, P. Chakraborty, I. Mitra, P. K. Ojha, S. Kar, and R. N. Das, J. Comput. Chem., 34, 1071 (2013).
I. Mitra, A. Saha, and K. Roy, Mol. Simul., 13, 1067 (2010).
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © 2017 J.-B. Tong, M. Bai, X. Zhao.
Rights and permissions
About this article
Cite this article
Tong, JB., Bai, M. & Zhao, X. QSAR study by the RASMS method of DABO derivatives as HIV-1 reverse transcriptase non-nucleoside inhibitors. J Struct Chem 58, 1418–1426 (2017). https://doi.org/10.1134/S0022476617070204
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022476617070204