Abstract
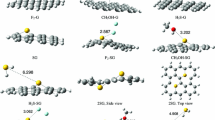
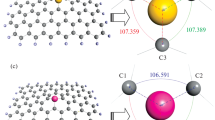
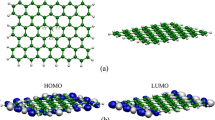
The effects of doping heteroatoms on the structure, electronic and adsorption properties of graphene are investigated using density functional theory calculations. Six different doped graphenes (with Al, B, Si, N, P, and S) are considered, and to obtain the interaction and adsorption properties, three sulfur-containing molecules (H2S, SO2, and thiophene) were interacted with selected graphenes. The adsorption energies (E ad) in the gas phase and solvents show the exothermic interaction for all complexes. The maximum E ad values are observed for aluminum doped graphene (AG) and silicon doped graphene (SiG), and adsorption energies in the solvent are not so different from those in the gas phase. NBO calculations show that the AG and SiG complexes have the highest E (2) interaction energies and simple graphene (G) and nitrogen doped graphene (NG) have the least E (2) energies. Population analyses show that doping heteroatoms change the energy gap. This gap changes more during the interaction and these changes make these structures useful in sensor devices. All calculated data confirm better adsorption of SO2 by graphenes versus H2S and thiophene. Among all graphenes, AG and then SiG are the best adsorbents for these structures.
Similar content being viewed by others
References
P. E. Lammert and V. H. Crespi, Phys. Rev. B, 61, 7308–7311 (2000).
F. Karlicky, R. Zboril, and M. Otyepka, J. Chem. Phys., 137, 34709–34712 (2012).
P. Rani and V. K. Jindal, RSC Adv., 3, 802–812 (2013).
E. V. Castro, K. S. Novoselov, S. V. Morozov, et al., Phys. Rev. Lett., 99, 216802–216805 (2007).
X. Yang, Y. Wang, X. V. Huang, et al., J. Mater. Chem., 21, 3448–3454 (2011).
S. C. Ohern, M. S. H. Boutilier, J. C. Idrobo, et al., Nano Lett., 14, 1234–1241 (2014).
P. Sun, M. Zhu, K. Wang, et al., ACS Nano, 7, 428–437 (2013).
D. C. Tanugi and J. C. Grossman, Nano Lett., 12, 3602–3608 (2012).
A. A. Koos, R. J. Nicholls, F. Dillon, et al., Carbon, 50, 2816–2823 (2012).
J. Liu, H. Liu, Y. Zhang, et al., Carbon, 49, 5014–5021 (2011).
F. H. Monteiro, D. G. Larrude, M. E. H. Maia da Costa, et al., J. Phys. Chem. C, 116, 3281–3285 (2012).
W. Yuan and G. J. Shi, Mater. Chem. A, 1, 10078–10091 (2013).
F. Hassani and H. Tavakol, Sens. Actuators B, 196, 624–630 (2014).
H. Tavakol and F. Hassani, Struct. Chem., 26, 151–158 (2015).
H. Tavakol and A. Mollaei-Renani, Struct. Chem., 25, 1659–1667 (2014).
A. L. E. Garcia, S. E. Baltazar, A. H. Romero, et al., J. Comput. Theor. Nanosci., 5, 221–229 (2008).
G. Shi, Y. Ding, and H. J. Fang, Comput. Chem., 33, 1328–1337 (2012).
E. C. Anota, A. R. Juarez, M. Castro, et al., J. Mol. Model., 19, 321–328 (2013).
H. Kishi, M. Tani, M. Sakaue, et al., J. Vac. Soc. Jpn., 55, 198–203 (2012).
I. G. Ayala and N. A. Cordero, J. Nanopart. Res., 14, 1071–1079 (2012).
A. N. Rudenko, F. J. Keil, M. I. Katsnelson, et al., Phys. Rev. B, 86, 075422 (2012).
M. Mirzaei and M. Yousefi, Superlattices Microstruct., 52, 1–7 (2013).
A. Ambrosetti and P. L. Silvestrelli, J. Phys. Chem. C, 115, 3695–3702 (2011).
L. Zhang, J. Niu, L. Dai, et al., Langmuir, 28, 7542–7550 (2012).
X. Gu, Y. Yang, Y. Hu, et al., RSC Adv., 4, 63189–63199 (2014).
Y. L. Wang, K. H. Su, and J. P. Zhang, Adv. Mater. Res., 463, 1488–1492 (2012).
M. J. Frisch, G. W. Trucks, H. B. Schlegel, et al. Gaussian 09, Revision A.1, Gaussian, Inc., Wallingford CT, USA (2009).
H. Tavakol, THEOCHEM, 954, 16–21 (2010).
Z. Javanshir, K. Mehrani, S. Ghammamy, et al., Bull. Korean Chem. Soc., 2, 1464–1466 (2008).
H. Tavakol, M. Esfandyari, S. Taheri, et al., Spectrochim. Acta A, 79, 574–582 (2011).
B. G. Johnson, P. M. W. Gill, and J. A. Pople, J. Chem. Phys., 98, 5612–5618 (1993).
C. W. Bauschlicher and H. T. Partridge, Chem. Phys. Lett., 240, 533–540 (1995).
H. Tavakol, T. Hadadi, and H. Roohi, J. Struct. Chem., 53, 649–658 (2012).
H. Tavakol, Int. J. Quantum Chem., 111, 3717–3724 (2011).
H. Tavakol, Mol. Simul., 36, 391–402 (2010).
J. D. Chai and M. Head-Gordon, Phys. Chem. Chem. Phys., 10, 6615–6620 (2008).
J. Antony and S. Grimme, Phys. Chem. Chem. Phys., 8, 5287–5293 (2006).
P. Jurecka, J. Cerny, P. Hobza, et al., J. Comput. Chem., 28, 555–569 (2007).
S. Mietrus and E. Scrocco, J. Chem. Phys., 55, 117–122 (1981).
E. D. Glendening, A. E. Reed, J. E. Carpenter, et al., NBO, Version 3.1 (2006).
N. M. Oboyle, A. L. Tenderholt, and K. M. Langner, J. Comput. Chem., 29, 839–845 (2008).
R. G. Parr, R. Donnelly, M. Levy, and W. E. Palke, J. Chem. Phys., 68, 3801–3808 (1978).
Author information
Authors and Affiliations
Corresponding author
Additional information
The text was submitted by the authors in English.
Zhurnal Strukturnoi Khimii, Vol. 58, No. 3, pp. 506-515, March-April, 2017.
Original Russian Text © 2017 S. Amir Aslanzadeh.
Rights and permissions
About this article
Cite this article
Amir Aslanzadeh, S. Effects of heteroatoms on the electronic, sensor, and adsorption properties of graphene. J Struct Chem 58, 479–488 (2017). https://doi.org/10.1134/S0022476617030088
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022476617030088