Abstract
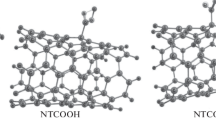
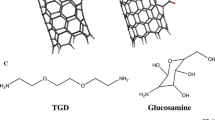
In this work, using quantum mechanics, the noncovalent interactions and two mechanisms of covalent functionalization of drug gentamicin with (5,5) COOH and COCl functionalized carbon nanotubes are studied. All of the calculations are performed using a hybrid density functional method (UB3LYP) in the solution phase. Quantum molecular descriptors for four possible modes of the noncovalent interaction are investigated. It is found that the binding of gentamicin with COOH (NCOOH) and COCl (NCOCl) functionalized carbon nanotubes is thermodynamically favorable. Among NCOOH and NCOCl, the first one has higher binding energy and can act as a suitable system for the drug gentamicin delivery within biological systems (noncovalent). COOH and COCl functionalized carbon nanotubes can bond to gentamicin via OH (COOH mechanism) and Cl (COCl mechanism) groups, respectively. The activation energies of four pathways in two mechanisms are calculated and compared with each other. It is specified that the COOH mechanism has an energy barrier higher than that of the COCl mechanism, being the reason for the suitability of the COCl mechanism for covalent functionalization.
Similar content being viewed by others
References
K. C. Popat, M. Eltgroth, T. J. LaTempa, C. A. Grimes, and T. A. Desai, Biomaterials, 28, 4880–4888 (2007).
D. Tomalia, L. Reyna, and S. Svenson, Biochem. Soc. Trans., 35, 61 (2007).
A. Chonn and P. R. Cullis, Curr. Opin. Biotechnol., 6, 698–708 (1995).
T. M. Allen and P. R. Cullis, Science, 303, 1818–1822 (2004).
M. Prato, K. Kostarelos, and A. Bianco, Acc. Chem. Res., 41, 60–68 (2007).
S. Wong, S. L. Yoong, A. Jagusiak, T. Panczyk, H. K. Ho, W. H. Ang, and G. Pastorin, Adv. Drug. Delivery Rev., 65, 1964–2015 (2013).
S. Sharifi, M. M. Hashemi, M. Mosslemin, and F. Mollaamin, J. Comput. Theor. Nanosci., 11, 1178–1183 (2014).
Z. Hosni, R. Bessrour, and B. Tangour, J. Comput. Theor. Nanosci., 11, 318–323 (2014).
N. Saikia and R. C. Deka, J. Mol. Model., 19, 215–226 (2013).
P. Prajongtat, S. Suramitr, M. P. Gleeson, K. Mitsuke, and S. Hannongbua, Monatsh. Chem., 144, 925–935 (2013).
Y. Lin, L. F. Allard, and Y.-P. Sun, J. Phys. Chem. B, 108, 3760–3764 (2004).
J. Azimov, S. Mamatkulov, N. Turaeva, B. Oxengendler, and S. S. Rashidova, J. Struct. Chem., 53, 829–834 (2012).
A. Star, Y. Liu, K. Grant, L. Ridvan, J. F. Stoddart, D. W. Steuerman, M. R. Diehl, A. Boukai, and J. R. Heath, Macromolecules, 36, 553–560 (2003).
J. Beheshtian, A. A. Peyghan, and Z. Bagheri, Monatsh. Chem., 143, 1623–1626 (2012).
G. Canto, E. Martínez-Guerra, and N. Takeuchi, Comp. Mater. Sci., 42, 322–328 (2008).
G. Dovbeshko, O. Fesenko, E. Obraztsova, K. Allakhverdiev, and A. Kaja, J. Struct. Chem., 50, 954–961 (2009).
M. Rajarajeswari, K. Iyakutti, and Y. Kawazoe, J. Mol. Model., 18, 771–781 (2012).
M. T. Baei, A. A. Peyghan, and M. Moghimi, Monatsh. Chem., 143, 1463–1470 (2012).
H. Li, J. He, Y. Zhao, G. Wang, and Q. Wei, J. Inorg. Organomet. Polym., 21, 890–892 (2011).
R. G. Parr, L. v. Szentpaly, and S. Liu, J. Am. Chem. Soc., 121, 1922–1924 (1999).
J. Fehir, J. Richard, and J. K. McCusker, J. Phys. Chem. A, 113, 9249–9260 (2009).
T. Lin, V. Bajpai, T. Ji, and L. Dai, Aust. J. Chem., 56, 635–651 (2003).
A. D. Becke, Phys. Rev. A, 38, 3098 (1988).
A. D. Becke, J. Chem. Phys., 98, 5648–5652 (1993).
C. Lee, W. Yang, and R. G. Parr, Phys. Rev. B, 37, 785 (1988).
M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalman, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery Jr., J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, O. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski, and D. J. Fox, Gaussian 09, Gaussian Inc., Wallingford CT (2009).
S. H. Vahidi, A. Morsali, and S. A. Beyramabadi, Comput. Theor. Chem., 994, 41–46 (2012).
S. A. Beyramabadi, A. Morsali, and A. Shams, J. Struct. Chem., 56, 243–249 (2015).
A. Morsali, F. Hoseinzade, A. Akbari, S. A. Beyramabadi, and R. Ghiasi, J. Solution Chem., 42, 1902–1911 (2013).
S. A. Beyramabadi, A. Morsali, S. H. Vahidi, M. Khoshkholgh, and A. Esmaeili, J. Struct. Chem., 53, 460–467 (2012).
S. A. Beyramabadi, H. Eshtiagh-Hosseini, M. R. Housaindokht, and A. Morsali, Organometallics, 27, 72–79 (2007).
H. Eshtiagh-Hosseini, S. A. Beyramabadi, M. Mirzaei, A. Morsali, A. Salimi, and M. Naseri, J. Struct. Chem., 54, 1063–1069 (2013).
A. Morsali, Int. J. Chem. Kinet., 47, 73–81 (2015).
R. Cammi and J. Tomasi, J. Comput. Chem., 16, 1449–1458 (1995).
J. Tomasi and M. Persico, Chem. Rev., 94, 2027–2094 (1994).
S. Dapprich, I. Komáromi, K. S. Byun, K. Morokuma, and M. J. Frisch, J. Mol. Struct.: THEOCHEM, 461, 1–21 (1999).
Author information
Authors and Affiliations
Corresponding author
Additional information
The text was submitted by the authors in English.
Zhurnal Strukturnoi Khimii, Vol. 58, No. 3, pp. 490-498, March-April, 2017.
Original Russian Text © 2017 A. Mansoorinasab, A. Morsali, M. M. Heravi, S. A. Beyramabadi.
Rights and permissions
About this article
Cite this article
Mansoorinasab, A., Morsali, A., Heravi, M.M. et al. Quantum mechanical study of carbon nanotubes functionalized with drug gentamicin. J Struct Chem 58, 462–470 (2017). https://doi.org/10.1134/S0022476617030064
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022476617030064