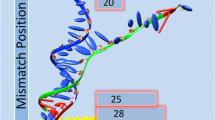
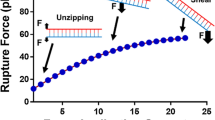
Abstract
The review is devoted to measurement methods of bond rupture forces in complex biological molecules, namely, the unwinding forces of a DNA double helix. Mechanical methods not affecting electromagnetically a system under study, which is especially significant for biological systems, are considered. We describe two main methods: atomic force microscopy and rupture event scanning. The latter is a new method also based on the mechanical action but it has a much simpler instrumental implementation. The capabilities of both methods are compared and they are shown to be promising to investigate chemical bond rupture forces in biological systems. The application of these methods to study the strength of chemical bonds is associated with overcoming numerous technical difficulties in both performance of measurements themselves and chemical modification of conjugated surfaces. We demonstrate the applicability of these methods not only for fundamental studies of the strength of chemical bonds determining the stability and the related possibility of functioning of three-dimensional biomolecular complexes, but also for the design of biosensors based on the mechanical effect (quartz crystal microbalance, QCM), e.g., with an opportunity of rapid analysis of DNA.
Similar content being viewed by others
References
S. K. Kufer, E. M. Puchner, H. Gumpp, T. Liedl, and H. E. Gaub, Science, 319, 594–596 (2008).
T. Strunz, K. Oroszlan, R. Schafer, and H.-J. Guntherodt, Proc. Natl. Acad. Sci. U.S.A., 96, 11277–11282 (1999).
F. Kienberger, G. Kada, H. Mueller, and P. Hinterdorfer, J. Mol. Biol., 34, 597–606 (2005).
S. J. Grabowski, Annu. Rep. Prog. Chem. C, 102, 131–165 (2006).
G. Binnig, C. F. Quate, and C. Gerber, Phys. Rev. Lett., 56, 930–933 (1986).
S. C. Minne, P. Flueckiger, H. T. Soh, and C. F. Quate, J. Vac. Sci. Technol., B, 13, No. 3, 1380–1385 (1995).
T. Junno, K. Deppert, L. Montelius, and L. Samuelson, Appl. Phys. Lett., 66, No. 26, 3627–3629 (1995).
Y. L. Lyubchenko and L. S. Shlyakhtenko, Proc. Natl. Acad. Sci., 94, No. 2, 496–501 (1997).
Y. C. Lee, H. J. Kim, K. S. Kim, S. Choi, S. W. Kim, H. K. Park, and Y. G. Eun, Microsc. Res. Tech., 78, No. 7, 569–576 (2015).
M. Leitner, N. Mitchell, M. Kastner, R. Schlapak, H. J. Gruber, and P. Hinterdorfer, ACS Nano, 5, No. 9, 7048–7054 (2011).
E. Rettler, S. Hoeppener, B. W. Sigusch, and U. S. Schubert, J. Mater. Chem. B, 1, No. 22, 2789–2806 (2013).
Q. Li, B. Doyran, L. W. Gamer, X. L. Lu, L. Qin, C. Ortiz, A. J. Grodzinsky, V. Rosen, and L. Han, J. Biomech., 48, No. 8, 1364–1370 (2015).
H. J. Butt, B. Cappella, and M. Kappl, Surf. Sci. Rep., 59, No. 1, 1–152 (2005).
F. L. Leite, C. C. Bueno, A. L. Da Róz, E. C. Ziemath, and O. N. Oliveira, Int. J. Mol. Sci., 13, No. 10, 12773–12856 (2012).
H. A. Kramers, Physica, 7, No. 4, 284–304 (1940).
G. I. Bell, Science, 200, No. 4342, 618–627 (1978).
E. Evans and K. Ritchie, Biophys. J., 72, No. 4, 1541 (1997).
O. H. Willemsen, M. M. Snel, K. O. Van Der Werf, B. G. De Grooth, J. Greve, P. Hinterdorfer, H. J. Gruber, H. Schindler, Y. van Kooyk, and C. G. Figdor, Biophys. J., 75, No. 5, 2220–2228 (1998).
S. Allen, J. Davies, A. C. Dawkes, M. C. Davies, J. C. Edwards, M. C. Parker, C. J. Roberts, J. Sefton, S. J. B. Tendler, and P. M. Williams, FEBS Lett., 390, No. 2, 161–164 (1996).
G. U. Lee, L. A. Chrisey, and R. J. Colton, Science, 266, No. 5186, 771–773 (1994).
M. E. Drew, A. Chworos, E. Oroudjev, H. Hansma, and Y. A. Yamakoshi, Langmuir, 26, No. 10, 7117–7125 (2009).
T. S. Tsapikouni and Y. F. Missirlis, Colloids Surf., B, 75, No. 1, 252–259 (2010).
F. Benedetti, Statistical Study of the Unfolding of Multimodular Proteins and their Energy Landscape by Atomic Force Microscopy, Doctoral Dissertation, École Polytechnique Fédérale De Lausanne (2012).
S. W. Han, S. Mieda, C. Nakamura, T. Kihara, N. Nakamura, and J. Miyake, J. Mol. Recognit., 24, No. 1, 17–22 (2011).
F. Schwesinger, R. Ros, T. Strunz, D. Anselmetti, H. J. Güntherodt, A. Honegger, L. Jermutus, L. Tiefenauer, and A. Plückthun, Proc. Natl. Acad. Sci., 97, No. 18, 9972–9977 (2000).
A. W. Flounders, D. L. Brandon, and A. H. Bates, Appl. Biochem. Biotech., 50, No. 3, 265–284 (1995).
J. Hoypierres, V. Dulong, C. Rihouey, S. Alexandre, L. Picton, and P. Thébault, Langmuir, 31, No. 1, 254–261 (2014).
J. Cho, N. Levy, A. Kirakosian, M. J. Comstock, F. Lauterwasser, J. M. Fréchet, and M. F. Crommie, J. Chem. Phys., 131, No. 3, 034707 (2009).
O. Cavalleri, C. Natale, M. E. Stroppolo, A. Relini, E. Cosulich, S. Thea, M. Novi, and A. Gliozzi, Phys. Chem. Chem. Phys., 2, No. 20, 4630–4635 (2000).
D. C. Klein, C. M. Stroh, H. M. van Es Jensenius, A. S. M. Kamruzzahan, A. Stamouli, H. J. Gruber, T. H. Oosterkamp, and P. Hinterdorfer, ChemPhysChem, 4, No. 12, 1367–1371 (2003).
J. Blass, M. Albrecht, B. L. Bozna, G. Wenz, and R. Bennewitz, Nanoscale, 7, No. 17, 7674–7681 (2015).
I. Y. Phang, N. Aldred, X. Y. Ling, J. Huskens, A. S. Clare, and G. J. Vancso, J. R. Soc., Interface, rsif20090127 (2009).
X. Han, M. Qin, H. Pan, Y. Cao, and W. Wang, Langmuir, 28, No. 26, 10020–10025 (2012).
A. R. Bizzarri and S. Cannistraro, Nanotechnology, 25, No. 33, 335102 (2014).
X. Wang, M. M. Shindel, S. W. Wang, and R. Ragan, Langmuir, 28, No. 19, 7417–7427 (2012).
P. Vermette, T. Gengenbach, U. Divisekera, P. A. Kambouris, H. J. Griesser, and L. Meagher, J. Colloid Interface Sci., 259, No. 1, 13–26 (2003).
F. Kühner, L. T. Costa, P. M. Bisch, S. Thalhammer, W. M. Heckl, and H. E. Gaub, Biophys. J., 87, No. 4, 2683–2690 (2004).
P. D. Pollheimer, P. Winklehner, M. Hölzl, B. Lackner, D. M. Schörkl, P. Hinterdorfer, and H. J. Gruber, Bioconjugate Chem., 17, No. 6, 1473–1481 (2006).
C. K. Riener, C. M. Stroh, A. Ebner, C. Klampfl, A. A. Gall, C. Romanin, Y. L. Lyubchenko, P. Hinterdorfer, and H. J. Gruber, Anal. Chim. Acta, 479, No. 1, 59–75 (2003).
E. Jauvert, E. Dague, M. Séverac, L. Ressier, A. M. Caminade, J. P. Majoral, and E. Trévisiol, Sens. Actuators, B, 168, 436–441 (2012).
V. K. Yadavalli, J. G. Forbes, and K. Wang, Langmuir, 22, No. 16, 6969–6976 (2006).
X. Zhang and V. K. Yadavalli, Anal. Chim. Acta, 649, No. 1, 1–7 (2009).
A. V. Krasnoslobodtsev, Y. Zhang, E. Viazovkina, A. Gall, C. Bertagni, and Y. L. Lyubchenko, Biophys. J., 108, No. 9, 2333–2339 (2015).
L. S. Shlyakhtenko, A. A. Gall, J. J. Weimer, D. D. Hawn, and Y. L. Lyubchenko, Biophys. J., 77, No. 1, 568–576 (1999).
K. Saal, V. Sammelselg, A. Lõhmus, E. Kuusk, G. Raidaru, T. Rinken, and A. Rinken, Biomol. Eng., 19, No. 2, 195–199 (2002).
E. Casero, M. Darder, D. J. Diaz, F. Pariente, J. A. Martin-Gago, H. Abruna, and E. Lorenzo, Langmuir, 19, No. 15, 6230–6235 (2003).
T. Das, P. K. Sharma, B. P. Krom, H. C. van der Mei, and H. J. Busscher, Langmuir, 27, No. 16, 10113–10118 (2011).
R. M. A. Sullan, J. K. Li, P. J. Crowley, L. J. Brady, and Y. F. Dufrêne, ACS Nano, 9, No. 2, 1448–1460 (2015).
A. Beaussart, A. E. Baker, S. L. Kuchma, S. El-Kirat-Chatel, G. A. O′Toole, and Y. F. Dufrêne, ACS Nano, 8, No. 10, 10723–10733 (2014).
A. P. Wiita, S. R. K. Ainavarapu, H. H. Huang, and J. M. Fernandez, Proc. Natl. Acad. Sci., 103, No. 19, 7222–7227 (2006).
H. Gumpp, E. M. Puchner, J. L. Zimmermann, U. Gerland, H. E. Gaub, and K. Blank, Nano Lett., 9, No. 9, 3290–3295 (2009).
W. Christenson, I. Yermolenko, B. Plochberger, F. Camacho-Alanis, A. Ros, T. P. Ugarova, and R. Ros, Ultramicroscopy, 136, 211–215 (2014).
Y. He, M. Lu, J. Cao, and H. P. Lu, ACS Nano, 6, No. 2, 1221–1229 (2012).
A. Noy, D. V. Vezenov, J. F. Kayyem, T. J. Maade, and C. M. Lieber, Chem. Biol., 4, No. 7, 519–527 (1997).
C. Ke, M. Humeniuk, S. Hanna, and P. E. Marszalek, Phys. Rev. Lett., 99, No. 1, 018302 (2007).
T. B. Zhang, C. L. Zhang, Z. L. Dong, and Y. F. Guan, Sci. Rep., 5, 9143 (2015).
M. Santosh and P. K. Maiti, J. Phys.: Condens. Matter, 21, No. 3, 034113 (2008).
R. Krautbauer, M. Rief, and H. E. Gaub, Nano Lett., 3, No. 4, 493–496 (2003).
E. Sengupta, Y. Yan, X. Wang, K. Munechika, and D. S. Ginger, ACS Nano, 8, No. 3, 2625–2631 (2014).
X. Wang, R. N. Sanderson, and R. Ragan, J. Phys. Chem. C, 118, No. 50, 29301–29309 (2014).
A. Ashkin, Phys. Rev. Lett., 24, No. 4, 156 (1970).
A. Ashkin, Phys. Rev. Lett., 25, No. 19, 1321 (1970).
J. R. Moffitt, Y. R. Chemla, S. B. Smith, and C. Bustamante, Biochemistry, 77, No. 1, 205 (2008).
L. P. Ghislain and W. W. Webb, Opt. Lett., 18, No. 19, 1678–1680 (1993).
H. Zhang and K. K. Liu, J. R. Soc., Interface, 5, No. 24, 671–690 (2008).
L. Sacconi, I. M. Tolić-Nørrelykke, C. Stringari, R. Antolini, and F. S. Pavone, Appl. Opt., 44, No. 11, 2001–2007 (2005).
K. C. Neuman, E. H. Chadd, G. F. Liou, K. Bergman, and S. M. Block, Biophys. J., 77, No. 5, 2856–2863 (1999).
J. E. Molloy and M. J. Padgett, Contemp. Phys., 43, No. 4, 241–258 (2002).
O. Otto, J. L. Gornall, G. Stober, F. Czerwinski, R. Seidel, and U. F. Keyser, J. Opt., 13, No. 4, 044011 (2011).
A. Sischka, R. Eckel, K. Toensing, R. Ros, and D. Anselmetti, Rev. Sci. Instrum., 74, No. 11, 4827–4831 (2003).
S. Heo, K. Kim, and Y. H. Cho, ChemPhysChem, 15, No. 8, 1573–1576 (2014).
E. J. Peterman, F. Gittes, and C. F. Schmidt, Biophys. J., 84, No. 2, 1308–1316 (2003).
B. Shergill, L. Meloty-Kapella, A. A. Musse, G. Weinmaster, and E. Botvinick, Dev. Cell, 22, No. 6, 1313–1320 (2012).
T. Stangner, C. Wagner, D. Singer, S. Angioletti-Uberti, C. Gutsche, J. Dzubiella, R. Hoffmann, and F. Kremer, ACS Nano, 7, No. 12, 11388–11396 (2013).
C. M. Cheng, Y. J. Lee, W. T. Wang, C. T. Hsu, J. S. Tsai, C. M. Wu, K. L. Ou, and T. S. Yang, Biochem. Biophys. Res. Commun., 404, No. 1, 297–301 (2011).
M. Castelain, S. Ehlers, J. Klinth, S. Lindberg, M. Andersson, B. E. Uhlin, and O. Axner, Eur. Biophys. J., 40, No. 3, 305–316 (2011).
M. Andersson, O. Björnham, M. Svantesson, A. Badahdah, B. E. Uhlin, and E. Bullitt, J. Mol. Biol., 415, No. 5, 918–928 (2012).
N. Mortezaei, B. Singh, J. Zakrisson, E. Bullitt, and M. Andersson, Biophys. J., 109, No. 1, 49–56 (2015).
M. Castelain, M. P. Duviau, A. Canette, P. Schmitz, P. Loubière, M. Cocaign-Bousquet, and M. Mercier-Bonin, PloS One, 11, No. 3, e0152053 (2016).
A. J. Crick, M. Theron, T. Tiffert, V. L. Lew, P. Cicuta, and J. C. Rayner, Biophys. J., 107, No. 4, 846–853 (2014).
G. Pobegalov, G. Cherevatenko, A. Alekseev, A. Sabantsev, O. Kovaleva, A. Vedyaykin, N. Morozova, D. Baitin, and M. Khodorkovskii, Biochem. Biophys. Res. Commun., 466, No. 3, 426–430 (2015).
F. N. Dultsev, V. P. Ostanin, and D. Klenerman, Langmuir, 16, 5036–5040 (2000).
F. N. Dultsev and E. A. Kolosovsky, Sens. Actuators, B, 143, 17–24 (2009).
F. N. Dultsev, E. A. Kolosovsky, and I. A. Mik, Langmuir, 28, 13793–13797 (2012).
B. Borovsky, B. L. Mason, and J. Krim, J. Appl. Phys., 88, No. 7, 4017–4021 (2000).
L. D. Landau and E. M. Lifshits, Theoretical Physics, vol. 7: Theory of Elasticity [in Russian], 4th ed., Nauka, Moscow (1987).
F. N. Dultsev and E. A. Kolosovsky, Sens. Actuators, B, 202, 454–460 (2014).
D. Eigler and E. Schweizer, Nature, 344, 524 (1990).
Y. Sugimoto, M. Abe, S. Hirayama, N. Oyabu, O. Custance, and S. Morita, Nat. Mater., 4, No. 2, 156–159 (2005).
T. Thundat, X. Zheng, G. Chen, and R. Warmack, Surf. Sci. Lett., 294, L939–L943 (1993).
F. N. Dultsev, E. A. Kolosovsky, I. A. Mik, A. A. Lomzov, and D. V. Pyshnyi, Langmuir, 30, 3795–3801 (2014).
F. N. Dultsev, E. A. Kolosovsky, M. A. Cooper, A. A. Lomzov, and D. V. Pyshnyi, Sens. Biosensing Res., 4, 11–15 (2015).
M. Mosayebi, A. A. Louis, J. P. Doye, and T. E. Ouldridge, ACS Nano, 9, No. 12, 11993–12003 (2015).
S. B. Smith, Y. Cui, and C. Bustamante, Science, 271, 796–799 (1996).
J. F. Marko and E. D. Siggia, Macromolecules, 28, 8759–8770 (1995).
H. Clausen-Schaumann, M. Rief, C. Tolksdorf, and H. E. Gaub, Biophys. J., 78, 1997–2007 (2000).
I. Rouzina and V. A. Bloomfield, Biophys. J., 890, 882–893 (2001).
B. Essevaz-Roulet, U. Bockelmann, and F. Heslot, Proc. Natl. Acad. Sci. U.S.A., 94, 11935–11949 (1997).
M. Rief, H. Clausen-Schaumann, and H. E. Gaub, Nat. Struct. Biol., 6, 346–349 (1999).
J. Morfill, F. Kuhner, K. Blank, R. A. Lugmaier, J. Sedlmair, and H. E. Gaub, Biophys. J., 93, No. 7, 2400–2409 (2007).
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © 2017 N. N. Kurus, F. N. Dultsev.
Translated from Zhurnal Strukturnoi Khimii, Vol. 58, No. 2, pp. 332–356, February–March, 2017.
Rights and permissions
About this article
Cite this article
Kurus, N.N., Dultsev, F.N. Measurement of the unwinding force of a DNA double helix. J Struct Chem 58, 315–339 (2017). https://doi.org/10.1134/S0022476617020135
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022476617020135