Abstract
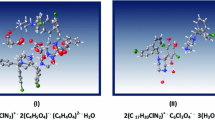
The crystal structure of {3-[2-(1,3-benzodioxol-5-yl)-7-methoxy-1-benzofuran-5-yl] propyl} diethylamine hydrohloride hydrate [C23H28NO4]+•[H2OCl]– is determined. The molecular geometrical parameters, frontier molecular orbital energies (HOMO, LUMO), their energy gap (ΔE), molecular electrostatic potential analysis of the compound are calculated by DFT/B3LYP at the 6-311G(d,p) level. The benzofuran and benzodioxo ring systems, except the diethylamine group, are essentially planar and a dihedral angle between the ring systems is 7.38(14)°. The compound crystallizes in the monoclinic space group P21/c, with a = 15.230(4) Å, b = 11.418(2) Å, c = 12.880(3) Å, β = 94.56(3)°, V = 2232.8(9) Å3, D calc = 1.297g/cm3, Z = 4. The hydrogen bonded Cl and H2O are self-assembled to form a supramolecular array of strong N–H…Cl and O–H…Cl bifurcated hydrogen bonds making tetramers which consist of a fused four-membered ring with a graph-set descriptor and a pseudo cyclic centrosymmetric R 2 2(8) ring motif. The hybrid dihalide-dihydrate clusters of [Cl2(H2O)2]2– are observed, too. The supramolecular crystal packing is consolidated by these bifurcated hydrogen bonds and the stacking of the sheet through strong π…π interactions. Moreover, the intra chain hydrogen bonds form intermolecular and intramolecular C–H…O hydrogen bonds, and the 1D supramolecular array is organized by C–H…π interactions. The contacts in the crystal structure are analyzed using the Hirshfeld surfaces computational method. The calculated geometrical parameters are in good agreement with the single crystal XRD data.
Similar content being viewed by others
References
R. J. Nevagi, S. N. Dighe, and S. N. Dighe, Eur. J. Med. Chem., 97, 561–581 (2015).
H. Khanam and Shamsuzzaman, Eur. J. Med. Chem., 97, 483–504 (2015).
S. E. Öztürk, Y. Akgül, and H. Anıl, Bioorg. Med. Chem., 16, 4431–4437 (2008).
S. E. Öztürk, T. Karayıldırım, and H. Anıl, Bioorg. Med. Chem., 19, 1179–1188 (2011).
Y. Marcus, Ion Solvation, Wiley, Chichester (1986).
D. T. Richens, The Chemistry of Aqua Ions, Wiley, Chichester (1997).
H. Ohtaki and T. Radnai, Chem. Rev., 93, 1157 (1993).
P. S. Lakshminarayanan, E. Suresh, and P. Ghosh, Angew. Chem., Int. Ed., 45, 3807 (2006).
R. Custelcean and M. G. Gorbunova, J. Am. Chem. Soc., 127, 16362 (2005).
D. D. Kemp and M. S. Gordon, J. Phys. Chem. A, 109, 7688 (2005).
W. H. Robertson and M. A. Johnson, Annu. Rev. Phys. Chem., 54, 173 (2003).
R. Ayala, J. M. Martínez, R. R. Pappalardo, and E. S. Marcos, J. Chem. Phys., 119, 9538 (2003).
D. Trzybin′ski and A. Sikorski, CrystEngComm, 15, 6808–6818 (2013).
M. Fujita (ed.), Structure and Bonding, vol. 96: Molecular Self-Assembly. Organic versus Inorganic Approaches, Springer (2000).
G. R. Desiraju, Crystal Engineering: The Design of Organic Solids, Elsevier, Amsterdam (1989).
J.-M. Lehn, Supramolecular Chemistry, Concepts and Perspectives, VCH, Weinheim (1995).
F. Li, T. H. Li, W. Su, S. Y. Gao, and R. Cao, Eur. J. Inorg. Chem., 1582–1587 (2006).
G. A. Jeffrey and S. Takagi, Acc. Chem. Res., 11, 264 (1978)
G. A. Jeffrey and H. Maluszynska, Int. J. Biol. Macromol., 4, 173 (1982)
G. A. Jeffrey and J. Mitra, J. Am. Chem. Soc., 106, 5546 (1984).
I. Rozas, I. Alkorta, and J. Elguero, J. Phys. Chem., 102, 9925–9932 (1998).
CrysAlisPro and CrysAlisRed, Agilent Technologies, Yarnton, Oxfordshire, England (2002).
O. V. Dolomanov, L. J. Bourhis, R. J. Gildea, J. A. K. Howard, and H. Puschmann, J. Appl. Crystallogr., 42, 339–341 (2009).
G. M. Sheldrick, Acta Crystallogr. A, 64, 112 (2008).
M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, J. A. Montgomery, J. T. Vreven, K. N. Kudin, J. C. Burant, J. M. Millam, S. S. Iyengar, J. Tomasi, V. Barone, B. Mennucci, M. Cossi, G. Scalmani, N. Rega, G. A. Petersson, H. Nakatsuji, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, M. Klene, X. Li, J. E. Knox, H. P. Hratchian, J. B. Cross, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, P. Y. Ayala, K. Morokuma, G. A. Voth, P. Salvador, J. J. Dannenberg, V. G. Zakrzewski, S. Dapprich, A. D. Daniels, M. C. Strain, O. Farkas, D. K. Malick, A. D. Rabuck, K. Raghavachari, J. B. Foresman, J. V. Ortiz, Q. Cui, A. G. Baboul, S. Clifford, J. Cioslowski, B. B. Stefanov, G. Liu, A. Liashenko, P. Piskorz, I. Komaromi, R. L. Martin, D. J. Fox, T. Keith, M. A. Al-Laham, C. Y. Peng, A. Nanayakkara, M. Challacombe, P. M. W. Gill, B. Johnson, W. Chen, M. W. Wong, C. Gonzalez, and J. A. Pople, Gaussian 03, Revision E. 01., Gaussian Inc., Pittsburgh, PA (2003).
A. Frisch, R. D. Dennington, T. A. Keith, J. Milliam, A. B. Nielsen, A. J. Holder, and J. Hiscocks, GaussView Reference, Version 4.0., Gaussian Inc., Pittsburgh (2007).
M. A. Spackman and D. Jayatilaka, CrystEngComm, 11, 19–32 (2009).
M. A. Spackman and J. J. McKinnon, CrystEngComm, 4, 378–392 (2002).
S. K. Wolff, D. J. Grimwood, J. J. McKinnon, M. J. Turner, D. Jayatilaka, and M. A. Spackman, CrystalExplorer (Version 3.0), University of Western Australia (2012).
D. Trzybinski and A. Sikorski, CrystEngComm, 15, 6808 (2013).
M. C. Etter, J. C. MacDonald, and J. Bernstein, Acta Crystallogr. B, 46, 256–262 (1990).
J. J. McKinnon, D. Jayatilaka, and M. A. Spackman, Chem. Commun., 3814–3816 (2007).
R. Chakrabarty, P. S. Mukherjee, and P. J. Stang, Chem. Rev., 111, No. 11, 6810–6918 (2011).
J. X. Dai, F. H. Wu, W. R. Yao, and Q. F. Zhang, Z. Naturforsch., 62b, 491–494 (2007).
J. Lundgren and I. Olovsson, Acta Crystallogr., 23, 971 (1967).
N. R. Babu, S. Subashchandrabose, M. S. A. Padusha, H. Saleem, and Y. Erdoğdu, Mol. Biomol. Spectrosc., 120, 314–322 (2014).
S. Murugavel, N. Manıkandan, D. Lakshmanan, K. Naveen, and P. T. Perumal, J. Chil. Chem. Soc., 60, 3 (2015).
J. J. McKinnon, M. A. Spackman, and A. S. Mitchell, Acta Crystallogr. B, 60, 627–668 (2004).
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © 2017 G. Yakalı, S. E. Öztürk, M. Aygün.
The text was submitted by the authors in English. Zhurnal Strukturnoi Khimii, Vol. 58, No. 2, pp. 321–331, February–March, 2017.
Rights and permissions
About this article
Cite this article
Yakalı, G., Öztürk, S.E. & Aygün, M. Strong hydrogen bonded supramolecular architecture in a crystal of the {3-[2-(1,3-benzodioxol-5-yl)-7-methoxy-1-benzofuran-5-yl] propyl} diethylamine cation with the hydrogen bonded chloride hydrate anion (halides) assembly: X-ray structure, DFT calculations, Hirshfeld surface analysis. J Struct Chem 58, 304–314 (2017). https://doi.org/10.1134/S0022476617020123
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022476617020123