Abstract
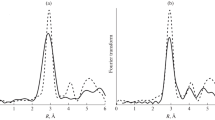
A local atomic structure around titanium positions in Ti-bearing hibonite (CaAl12O19) has been studied. The structural models of substitution of different substitution defects Ti–Al in hibonite by titanium atoms have been considered. Optimization of structural models of hibonite has been done by means of density functional theory calculations using pseudopotential approximation as implemented in VASP 5.3 code. Gibbs free energies analysis has shown that models of substitution of M2 and M4 aluminum positions by titanium atoms are the most probable. For the most probable structural models of Ti-bearing hibonite theoretical X-ray absorption near-edge structure (XANES) spectra near the titanium K edge have been calculated. Significant differences in theoretical XANES spectra calculated for different structural models with non-optimized and optimized atomic structure have been demonstrated. Changes in the intensity of pre-edge structure of TiK XANES spectra for different substitution models of aluminum by titanium have been observed which relate to different titanium coordination in structural models. Energy shift of spectral features towards lower energy for optimized models implies increase of interatomic distances in local surroundings of Ti absorbing atoms.
Similar content being viewed by others
References
Y. Amelin, A. N. Krot, A. A. Ulyanov, and I. D. Hutcheon, Science, 297, 1678 (2002).
A. T. Anderson, A. V. Crewe, J. R. Goldsmith, et al., Science, 167, 587 (1970).
H. Curien, C. Guillemin, J. Orcel, and M. Sternberg, C. R. Acad. Sci., Paris, 2845 (1956).
K. Kato and H. Saalfeld, Neues Jahrb. Mineral., Abh., 109, 192 (1968).
A. A. Utsunomiya, K. Tanaka, H. Morikawa, et al., J. Solid State Chem., 75, 197 (1988).
V. V. Bermanec, D. Holtstam, D. Sturman, et al., Can. Mineral., 34, 1287 (1996).
A. M. Hofmeister, B. Wopenka, and A. J. Locock, Geochim. Cosmochim. Acta, 68, 4485 (2004).
M. Nagashima, T. Armbruster, and T. Hainschwang, Mineral. Mag., 74, 871 (2010).
R. G. Burns and V. M. Burns, J. Geophys. Res., 89, 313 (1984).
J. R. Beckett, D. Live, F.-D. Tsay, et al., Geochim. Cosmochim. Acta, 52, 1479 (1988).
P. M. Doyle, P. F. Schofield, A. J. Berry, et al., Am. Mineral., 99, 1369 (2014).
G. Bunker, Introduction to XAFS. A Practical Guide to X-ray Absorption Fine Structure Spectroscopy, Cambridge University Press, UK (2010).
G. Kresse and J. Furthmüller, Phys. Rev. B, 54, No. 16, 11169 (1996).
J. P. Perdew, K. Burke, and M. Ernzerhof, Phys. Rev. Lett., 77, 3865 (1996).
D. M. Bylander, L. Kleinmann, and S. Lee, Phys. Rev. B, 42, 1394 (1990).
E. L. Frank, W. J. Daniel, W. Boisvert, et al., Conf. Proc. (2010).
J. J. Rehr, J. J. Kas, F. D. Vila, et al., Phys. Chem. Chem. Phys., 12, 5503 (2010).
J. J. Rehr, J. J. Kas, M. P. Prange, et al., C. R. Phys., 10, No. 6, 548 (2009).
Y. Joly, Phys. Rev. B, 63, 125120 (2001).
S. A. Guda, A. A. Guda, M. A. Soldatov, et al., J. Chem. Theor. Comput., 11, No. 9, 4512–4521 (2015).
A. N. Kravtsova, S. A. Suchkova, M. B. Fayn, and A. V. Soldatov, J. Struct. Chem., 57, No. 3, 491 (2016).
A. N. Kravtsova, K. A. Lomachenko, A. V. Soldatov, et al., J. Electron Spectrosc. Relat. Phenom., 195, 189 (2014).
I. S. Rodina, A. N. Kravtsova, A. V. Soldatov, and A. J. Berry, Opt. Spectrosc., 111, No. 6, 936 (2011).
I. S. Rodina, A. N. Kravtsova, A. V. Soldatov, et al., Opt. Spectrosc., 115, No. 6, 858 (2013).
A. Bianconi, M. Dell Ariccia, A. Gargano, and C. R. Natoli, in: Bond Length Determination Using XANES, EXAFS and Near Edge Structure, A. Bianconi, A. Incoccia, and S. Stipcich (eds.), Springer, Berlin (1987), p. 57.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Zhurnal Strukturnoi Khimii, Vol. 57, No. 7, pp. 1445-1452, September-October, 2016.
Rights and permissions
About this article
Cite this article
Pankin, I.A., Kravtsova, A.N., Polozhentsev, O.E. et al. Modelling of substitutional defects in the structure of Ti-bearing hibonite. J Struct Chem 57, 1369–1376 (2016). https://doi.org/10.1134/S0022476616070106
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022476616070106