Abstract
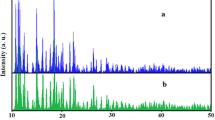
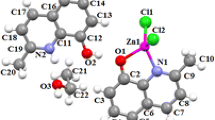
A new titanium complex [Ti(Me–Q)2(Cl)2] (1) is prepared by reacting titanium tetrachloride with 2-methyl-8-hydroxyquinoline in a fast and facile process. The complex is fully characterized based on its 1H and 13C NMR, IR, and UV spectra and elemental analysis. The prepared nanostructured compound is synthesized by the sonochemical method. This new nanostructure is characterized by scanning electron microscopy (SEM), powder X-ray diffraction (XRD), IR spectroscopy, and elemental analysis. Thermal stability of single crystalline and nanosize samples of the prepared compound is studied by thermal gravimetric (TG) and differential thermal analysis (DTA). The prepared complexes both bulk and nanosized are utilized as a precursor for the preparation of TiO2 nanoparticles by direct thermal decomposition at 600°C in air. The morphology and size of TiO2 nanoparticles are determined by SEM, powder XRD, and IR spectroscopy and the results show that the TiO2 nanoparticle size depends on the initial particle size of 1. Photoluminescence (PL) properties of the nanostructured and crystalline bulk prepared complex and their TiO2 nanoparticle cores are investigated.
Similar content being viewed by others
References
J. Shinar, Organic Light-Emitting Devices, Springer, New York (2003).
R. D. Miller and E. A. Chandross, Chem. Rev., 110, 1/2 (2010).
N. Koch, Chem. Phys. Chem., 8, 1438–1455 (2007).
R. H. Holm and M. J. O’Connor, Prog. Inorg. Chem., 14, 241–401(1971).
A. D. Garnovskii, A. L. Nivorozhkin, and V. I. Minkin, Coord. Chem. Rev., 126, 1–69 (1993).
Y. Qin, C. Pagba, P. Piotrowiak, and F. Jakle, J. Am. Chem. Soc., 126, 7015–7018 (2004).
M. Brinkmann, B. Fite, S. Pratontep, and C. Chaumont, Chem. Mater., 16, 4627–4633 (2004).
N. M. Shavaleev, H. Adams, J. Best, R. Edge, S. Navaratnam, and J. A. Weinstein, Inorg. Chem., 45, 9410–9415 (2006).
Y. Hamada, T. Sano, M. Fujita, T. Fujii, Y. Nishio, and K. Shibata, Jpn. J. Appl. Phys., 32, L511–L513 (1993).
S. M. Kim, J. S. Kim, D. M. Shin, Y. K. Kim, and Y. Ha, Bull. Korean Chem. Soc., 22, 743–747 (2001).
P. F. Wang, Z. R. Hong, Z. Y. Xie, S. W. Tong, O. Wong, C. S. Lee, N. B. Wong, L. S. Hung, and S. T. Lee, Chem. Commun., 14, 1664/1665 (2003).
T. Yu, W. Su, W. Li, Z. Hong, R. Hua, M. Li, B. Chu, B. Li, Z. Zhang, and Z. Z. Hu, Inorg. Chim. Acta, 359, 2246–2251 (2006).
H. T. Shi, L. M. Qi, J. M. Ma, and H. M. Cheng, J. Am. Chem. Soc., 125, 3450/3451 (2003).
H. Zhang, D. R. Yang, D. S. Li, X. Y. Ma, S. Z. Li, and D. L. Que, Cryst. Growth. Des., 5, 547–550 (2005).
D. B. Kuang, A. W. Xu, Y. P. Fang, H. Q. Liu, C. Frommen, and D. Fenske, Adv. Mater., 15, 1747–1750 (2003).
F. Kim, S. Connor, H. Song, T. Kuykendall, and P. D. Yang, Angew. Chem. Int. Ed., 43, 3673–3677 (2004).
J. Chen, T. Herricks, and Y. Xia, Angew. Chem. Int. Ed., 44, 2589–2592 (2005).
D. Horn and J. Rieger, Angew. Chem. Int. Ed., 40, 4330–4361 (2001).
X. F. Shen and X. P. Yan, Angew. Chem. Int. Ed., 46, 7659–7663 (2007).
D. E. Zhang, X. J. Zhang, X. M. Ni, H. G. Zheng, and D. D. Yang, J. Magn. Magn. Mater., 292, 79–82 (2005).
J. Wang, M. S. Gudiksen, X. Duan, Y. Cui, and C. M. Lieber, Science, 293, 1455–1457 (2001).
W. Lu, X. Qin, Y. Luo, G. Chang, and X. Sun, Microchim. Acta, 175, 355–359 (2011).
M. Bruchez, M. Moronne, P. Gin, S. Weiss, and A. P. Alivisatos, Science, 281, 2013–2016 (1998).
C. A. Mirkin, R. L. Letsinger, R. C. Mucic, and J. J. Storhoff, Nature, 382, 607–609 (1996).
M. S. Yeh, Y. S. Yang, Y. P. Lee, H. F. Lee, Y. H. Yeh, and C. S. Yeh, J. Phys. Chem. B, 103, 6851–6857 (1999).
E. Shahriari, W. M. M. Yunus, and E. Saion, Braz. J. Phys., 40, 256–260 (2010).
A. A. Ponce and K. J. Klabunde, J. Mol. Catal. A: Chem., 225, 1–6 (2005).
N. Iranpoor, H. Firouzabadi, A. Safavi, S. Motevalli, and M. M. Doroodmand, Appl. Organomet. Chem., 26, 417–424 (2012).
K. Kowlgi, U. Lafont, M. Rappolt, and G. Koper, J. Colloid Interface Sci., 372, 16–23 (2012).
Z. Khan, S. A. Al-Thabaiti, A. Y. Obaid, and A. O. Al-Youbi, Colloids Surf. B, 82, 513–517 (2011).
D. D. Perrin, W. L. F. Armarego, and D. R. Perrin, Purification of Laboratory Chemicals, 2nd ed., Springer, New York (1980).
K. S. Suslick, S. B. Choe, A. A. Cichowlas, and M. W. Grinstaff, Nature, 353, 414–416 (1991).
W. F. Zeng, Y. S. Chen, M. Y. Chiang, S. S. Chern, and C. P. Cheng, Polyhedron, 21, 1081–1087 (2002).
J. H. He, T. H. Wu, C. L. Hsin, K. M. Li, L. J. Chen, Y. L. Chueh, L. J.Chou, and Z. L. Wang, Small, 2, 116–120 (2006).
P. Gupta and M. Ramrakhiani, Open. Nanosci. J., 3, 15–19 (2009).
J. Huang, X. Wang, and A. J. Jacobson, J. Mater. Chem., 13, 191–196 (2003).
P. Jiang, W. Zhu, Z. Gan, W. Huang, J. Li, H. Zeng, and J. Shi, J. Mater. Chem., 19, 4551–4556 (2009).
L. A. Moreno, J. Visualized Exp., 63, e3066 (2012); doi: 10.3791/3066.
A. S. Juarez and A. Ortiz, J. Electrochem. Soc., 147, 3708–3717 (2000).
L. C. Nehru, V. Swaminathan, and C. Sanjeeviraja, Am. J. Mater. Sci., 2, 6–10 (2012).
G. Ramakrishna and H. N. Ghosh, Langmuir, 19, 505–508 (2003).
M. M. Rahman, K. M. Krishna, T. Soga, T. Jimbo, and M. Umeno, J. Phys. Chem. Solids, 60, 201–210 (1999).
E. Pelizzetti and C. Minero, Elecrochim. Acta, 38, 47–55 (1993).
B. Li, X. Wang, M. Yan, and L. Li, Mater. Chem. Phys., 78,184–188 (2002).
Y. Kolenko, B. R. Churagulov, M. Kunst, L. Mazerolles, and C. Colbeau-Justin, Appl. Catal. B, 54, 51–58 (2004).
W. Zhou, Q. Cao, and S. Tang, Powder Technol., 168, 32–36 (2006).
M. Kowshik, W. Vogel, J. Urban, S. K. Kulkarni, and K. M. Paknikar, Adv. Mater., 14, 815–818 (2002).
S. L. Xiong, B. J. Xi, D. C. Xu, C. M. Wang, X. M. Feng, H. Y. Zhou, and Y. T. Qian, J. Phys. Chem. C, 111, 16761–16767 (2007).
X. Liu, X. Wu, H. Cao, and R. P. H. Chang, J. Appl. Phys., 95, 3141–3147 (2004).
G. Mills, Z. G. Li, and D. Meisel, J. Phys. Chem., 92, 822–828 (1988).
Author information
Authors and Affiliations
Corresponding author
Additional information
The text was submitted by the authors in English. Zhurnal Strukturnoi Khimii, Vol. 57, No. 4, pp. 822-830, May-June, 2016.
Rights and permissions
About this article
Cite this article
Jodaian, V., Langeroodi, N.S. & Najafi, E. Structure and photoluminescence properties of TiO2 nanoparticles synthesized from a novel luminescent nano-titanium complex. J Struct Chem 57, 784–792 (2016). https://doi.org/10.1134/S0022476616040235
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022476616040235