Abstract
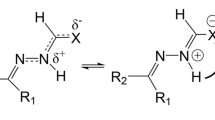
The reaction mechanism of the N–N bond cleavage in Ta(IV) hydrazido and hydrazidium complexes is studied using density functional theory. The N–N bond cleavage in Ta(IV) hydrazidium generates formal Ta(IV) nitridyl. The N–N bond cleavage in Ta(V) hydrazido gives terminal Ta(V) nitrido species. In the tetrahydrofuran solvent, terminal Ta(V) nitrido dimerizes through a one-step direct pathway leading to the [Ta(V),Ta(V)] bis(μ-nitrido) product. Two Ta–N bonds form simultaneously between the Ta center of one molecule and the terminal N atom of another. In the toluene solvent, there are two pathways of H atom abstraction and protonation producing mononuclear Ta(V) parent imide. The former consists of three steps originated from formal Ta(IV) nitridyl. The latter is unfavorable with terminal Ta(V) nitrido as the precursor.
Similar content being viewed by others
References
S. Hinrichsen, H. Broda, C. Gradert, et al., Annu. Rep. Prog. Chem., Sect. A: Inorg. Chem., 108, 17–47 (2012).
D. V. Yandulov and R. R. Schrock, Science, 301, 76–78 (2003).
R. R. Schrock, Acc. Chem. Res., 38, 955–962 (2005).
S. A. DiFranco, R. J. Staplesa, and A. L. Odom, Dalton Trans., 42, 2530–2539 (2013).
T. Shima, S. W. Hu, G. Luo, et al., Science, 340, 1549–1552 (2013).
M. D. Fryzuk, Science, 340, 1530/1531 (2013).
M. D. Fryzuk, Chem. Commun., 49, 4866–4868 (2013).
T. M. Figg, P. L. Holland, and T. R. Cundari, Inorg. Chem., 51, 7546–7550 (2012).
K. Umehara, S. Kuwata, and T. Ikariya, J. Am. Chem. Soc., 135, 6754–6757 (2013).
I. A. Tonks, A. C. Durrell, H. B. Gray, et al., J. Am. Chem. Soc., 134, 7301–304 (2012).
A. J. Keane, P. Y. Zavalij, and L. R. Sita, J. Am. Chem. Soc., 135, 9580–9583 (2013).
a)_B. L. Yonke, J. P. Reeds, P. Y. Zavalij, and L. R. Sita, Angew. Chem., Int. Ed., 50, 12342–12346 (2011)
J. P. Reeds, B. L. Yonke, P. Y. Zavalij, and L. R. Sita, J. Am. Chem. Soc., 133, 18602–18605 (2011)
P. P. Fontaine, B. L. Yonke, P. Y. Zavalij, and L. R. Sita, J. Am. Chem. Soc., 132, 12273–12285 (2010).
a)_J. Sgrignani, D. Franco, and A. Magistrato, Molecules, 16, 442–465 (2011)
B. L. Tran, B. Pinter, A. J. Nichols, et al., J. Am. Chem. Soc., 134, 13035–13045 (2012).
M. J. Frisch et. al., Gaussian 09, Revision B.01, Gaussian Inc., Wallingford, CT (2009).
A. D. Becke, J. Chem. Phys., 98, 5648–5652 (1993).
A. D. Becke, J. Chem. Phys., 104, 1040–1046 (1996).
C. Lee, W. Yang, and R. G. Parr, Phys. Rev. B, 37, 785–789 (1998).
P. J. Hay and W. R. Wadt, J. Chem. Phys., 82, 299–310 (1985).
W. R. Wadt and P. J. Hay, J. Chem. Phys., 82, 284–298 (1985).
O. Tapia, J. Math. Chem., 10, 139–181 (1992).
J. Tomasi and M. Persico, Chem. Rev., 94, 2027–2094 (1994).
J. Tomasi, B. Mennucci, and R. Cammi, Chem. Rev., 105, 2999–3093 (2005).
A. Schaefer, C. Huber, and R. Ahlrichs, J. Chem. Phys., 100, 5829–5835 (1994).
T. Ide, D. Takeuchi, and K. Osakada, Chem. Commun., 48, 278–280 (2012).
W. Zhang, Y. Tang, M. Lei, et al., Inorg. Chem., 50, 9481–9490 (2011).
a)_N. Lu, L. Meng, D. Z. Chen, et al., J. Phys. Chem. A, 116, 670–679 (2012)
N. Lu and H. Wang, Dalton Trans., 42, 13931–13939 (2013).
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © 2016 N. Lu, H. Wang.
Rights and permissions
About this article
Cite this article
Lu, N., Wang, H. A theoretical investigation on the N–N bond cleavage in Ta(IV) hydrazidium and Ta(V) hydrazido complexes. J Struct Chem 57, 47–53 (2016). https://doi.org/10.1134/S0022476616010066
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022476616010066