Abstract
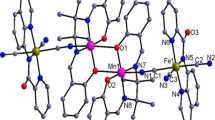
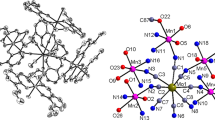
By employing trans-dicyano or pentacyanometalate as building block and using a bicompartimental Schiffbase based manganese(III) compound as assemble segment, two new cyanide-bridged heterometallic Fe(III)–Mn(III) complexes {[Mn(L)(H2O)][Febpb(CN)2]}·2CH3OH (1) and {[Mn(L)(H2O)]2··[Fe(CN)5NO]} (2) (bpb2– = 1,2-bis(pyridine-2-carboxamido)benzenate, L = N,N'-ethylene-bis(3-ethoxysalicylideneiminate) have been synthesized and characterized by elemental analysis, IR spectroscopy and X-ray structure determination. Single X-ray diffraction analysis reveals binuclear FeMn and trinuclear FeMn2 structure, respectively, in which the cyanide precursor acts as mono- or bidentate ligand to connect the Mn(III) Schiff-base unit(s). Furthermore, these two complexes are self-complementary through coordinated aqua ligands from one complex and the free O4 compartments from the neighboring complex, giving dimeric and 1D single chain supramolecular structure. Investigation of the magnetic susceptibility of 1 reveals weak antiferromagnetic coupling between the adjacent Mn(III) ions. Based on the binuclear FeMn model, best fit of the magnetic susceptibilities of 1 leads to the magnetic coupling constants J =–1.37 cm–1 and zJ′ =–0.72 cm–1 (1).
Similar content being viewed by others
References
A. Cornia, M. Mannini, P. Sainctavit, et al., Chem. Soc. Rev., 40, 3076 (2011).
P. Dechambenoit and J. R. Long, Chem. Soc. Rev., 40, 3249 (2011).
S. Sanvito, Chem. Soc. Rev., 40, 3336 (2011).
L. Sorace, C. Benelli, and C. D. Gatteschi, Chem. Soc. Rev., 40, 3092 (2011).
K. R. Dunbar and R. A. Heintz, Prog. Inorg. Chem., 45, 283 (1997).
M. Verdaguer, A. Bleuzen, V. Marvaud, et al., Coord. Chem. Rev., 190-192, 1023 (1999).
M. Ohba and H. Okawa, Coord. Chem. Rev., 198, No. 1, 313 (2000).
J. Cernák, M. Orendác, I. Potocnák, et al., Coord. Chem. Rev., 224, Nos. 1/2, 51 (2002).
M. Shatruk, C. Avendano, and K. R. Dunbar, Prog. Inorg. Chem., 56, 155 (2009).
O. Sato, T. Iyoda, A. Fujishima, et al., Science, 271, 49 (1996).
O. Sato, T. Iyoda, A. Fujishima, et al., Science, 272, 704 (1996).
A. Bleuzen, V. Marvaud, C. Mathonière, et al., Inorg. Chem., 48, No. 8, 3453 (2009).
V. Martínez, I. Boldog, A. B. Gaspar, et al., Chem. Mater., 22, No. 14, 4271 (2010).
C. Bartual-Murgui, L. Salmon, A. Akou, et al., Chem. Eur. J., 18, No. 2, 507 (2012).
I. R. Jeon, S. Calancea, A. Panja, et al., Chem. Sci., 4, 2463 (2013).
T. Shiga, G. N. Newton, J. S. Mathieson, et al., Dalton Trans., 39, No. 20, 4730 (2010).
M. X. Yao, Q. Zheng, X. M. Cai, et al., Inorg. Chem., 51, No. 4, 2140 (2012).
C. M. Liu, G. R. Xiong, D. Q. Zhang, et al., J. Am. Chem. Soc., 132, No. 12, 4044 (2010).
M. Gruselle, C. Train, K. Boubekeur, et al., Coord. Chem. Rev., 250, Nos. 19/20, 2491 (2006).
D. P. Zhang, Y. Z. Bian, J. Qin, et al., Dalton Trans., 43, No. 3, 945 (2014).
D. E. Freedman, D. M. Jenkins, A. T. Iavarone, et al., J. Am. Chem. Soc., 130, No. 10, 2884 (2008).
A. L. Goodwin, B. J. Kennedy, and C. Kepert, J. Am. Chem. Soc., 131, No. 18, 6334 (2009).
Z. H. Ni, H. Z. Kou, L. F. Zhang, et al., Angew. Chem., Int. Ed., 44, No. 47, 7742 (2005).
L. M. Toma, R. Lescouëzec, J. Pasan, et al., J. Am. Chem. Soc., 128, No. 14, 4842 (2006).
D. P. Zhang, L. F. Zhang, Y. T. Chen, et al., Chem. Commun., 46, No. 20, 3550 (2010).
H. Miyasaka, A. Saitoh, and S. Abe, Coord. Chem. Rev., 251, No. 21-24, 2622 (2007).
D. P. Zhang, H. L. Wang, Y. T. Chen, et al., Inorg. Chem., 48, No. 23, 11215 (2009).
D. P. Zhang, L. F. Zhang, X. Chen, et al., Inorg. Chim. Acta., 377, No. 1, 165 (2011).
H. Miyasaka, N. Matsumoto, H. Okawa, et al., J. Am. Chem. Soc., 118, No. 1, 981 (1996).
M. Ray, R. Mukherjee, J. F. Richardson, et al., J. Chem. Soc., Dalton Trans., No. 16, 2451 (1993).
G. M. Sheldrick, SHELX-97, Universität Göttingen, Germany (1997).
B. E. Myers, L. Berger, and S. Friedberg, J. Appl. Phys., 40, No. 3, 1149 (1969).
C. J. O’Connor, Prog. Inorg. Chem., 29, 203 (1982).
S. Nastase, C. Maxim, M. Andruh, et al., Dalton Trans., 40, No. 18, 4898 (2011).
D. P. Zhang, H. L. Wang, L. J. Tian, et al., Cryst. Growth Des., 9, No. 9, 3989 (2009).
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © 2015 H. Zhang, L. Kong, and D. Zhang.
The text was submitted by the authors in English. Zhurnal Strukturnoi Khimii, Vol. 56, No. 8, pp. 1594-1600, December, 2015.
Rights and permissions
About this article
Cite this article
Zhang, H., Kong, L. & Zhang, D. Cyanide-bridged bi- and trinuclear heterobimetallic Fe(III)–Mn(III) complexes: Synthesis, crystal structures and magnetic properties. J Struct Chem 56, 1533–1539 (2015). https://doi.org/10.1134/S0022476615080119
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022476615080119