Abstract
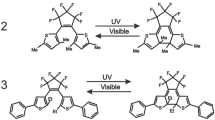
DFT calculations are employed to investigate the effects of the addition of a photoisomerizable stilbene unit to Aida′s molecular scissors on relative energies, dipole moments, and kinetic stability according to HOMO-LUMO energy gaps and amplitude of the open-close motion of blade moieties. The most obvious finding emerging from this study is the coming into existence of a new pair of molecular scissors operated by two photoswitchable units. Based on photoisomerization of azobenzene and stilbene units, four conformations appear for these new molecular scissors: cis–cis, cis–trans, trans–cis, and trans–trans. The HOMO-LUMO energy gaps promise that all isomers are kinetically stable. The other important finding is that in these new molecular scissors the dihedral angle between the two blade moieties can be controlled and measured through the open-close motion and the blade parts can adopt two middle states in addition to open-close forms.
Similar content being viewed by others
References
M. C. T. Fyfe, P. T. Glink, S. Menzer, J. F. Stoddart, A. J. P. White, and D. J. Williams, Angew. Chem. Int. Ed., 36, 2068–2070 (1997).
E. L. Eliel and S. H. Wilen, Stereochemistry of Organic Compounds, Wiley-Interscience, New York (1994).
C. Dugave and L. Demange, Chem. Rev., 103, 2475–2532 (2003).
D. H. Waldeck, Chem. Rev., 91, 415–436 (1991).
H. Meier, Angew. Chem., Int. Ed., 31, 1399–1420 (1992).
J. Saltiel, D. F. Sears Jr., D.-H. Ko, and K.-M. Park, in: CRC Handbook of Organic Photochemistry and Photobiology, W. M. Horspool and P.-S. Song (eds.), CRC, Boca Raton (1995). pp. 3–15.
H. Suginome, in: CRC Handbook of Organic Photochemistry and Photobiology W. M. Horspool and P.-S. Song (eds.), CRC, Boca Raton (1995). pp. 824–840.
E. R. Kay, D. A. Leigh, and F. Zerbetto, Angew. Chem., Int. Ed., 46, 72–191 (2007).
E. Durgun and J. C. Grossman, J. Phys. Chem. Lett., 4, 854–860 (2013).
V. Balzani, A. Credi, S. Silvi, and M. Venturi, Chem. Soc. Rev., 35, 1135–1149 (2006).
T. Muraoka, K. Kinbara, Y. Kobayashi, and T. Aida, J. Am. Chem. Soc., 125, 5612/5613 (2003).
T. Muraoka, K. Kinbara, and T. Aida, Nature, 440, 512/513 (2006).
F. M. Raymo, Angew. Chem., Int. Ed., 45, 5249–5251 (2006).
E. Merino and M. Ribagorda, Beilstein J. Org. Chem., 8, 1071–1090 (2012).
T. Muraoka and K. Kinbara, J. Photochem. Photobiol. C, 13, 136–147 (2012).
Y. B. Zheng, Q. Hao, Y.-W. Yang, B. Kiraly, I. Chiang, and T. J. Huang, J. Nanophotonics, 4, 4 (2010).
K. Kinbara and T. Aida, Chem. Rev., 105, 1396 (2005).
A. D. Becke, Phys. Rev. A, 38, 3098–3100 (1988).
A. D. Becke, J. Chem. Phys., 98, 5648–5652 (1993).
C. Lee, W. Yang, and R. G. Parr, Phys. Rev. B, 37, 785–789 (1988).
P. C. Hariharan and J. A. Pople, Mol. Phys., 27, 209–214 (1974).
J. Pople, Chem. Phys., 77, 3654–3665 (1982).
W. J. Hehre, L. Radom, P. v. R. Schleyer, and J. A. Pople, J. Comput. Chem., 7, 379 (1986).
M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman Jr., J. A. Montgomery, T. Vreven, K. N. Kudin, J. C. Burant, J. M. Millam, S. S. Iyengar, J. Tomasi, V. Barone, B. Mennucci, M. Cossi, G. Scalmani, N. Rega, P. G. A. Petresson, H. Nakatsuji, M. Hada, M. Ehara, K. Toyota, H. Nakai, M. Klene, X. Li, J. E. Knox, H. P. Hratchian, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, P. Y. Ayala, K. Morokuma, G. A. Voth, P. Salvador, J. J. Dannenberg, V. G. Zakrzewski, S. Dapprich, A. D. Daniels, M. C. Strain, O. Farkas, D. K. Malick, A. D. Rabuck, K. Raghavachari, J. B. Foresman, J. V. Ortiz, Q. Cui, A. G. Baboul, S. Clifford, J. Cioslowski, B. B. Stefanov, G. Liu, A. Liashenko, P. Piskorz, I. Komaromi, R. L. Martin, D. J. Fox, T. Keith, M. A. Al-Laham, C. Y. Peng, A. Nanayakkara, M. Challacombe, P. M. W. Gill, B. Johnson, W. Chen, M. W. Wong, C. Gonzalez, and J. A. Pople, Gaussian03, revision C.02, Gaussian Inc, Wallingford (2004).
T. Schultz, J. Quenneville, B. Levine, A. Toniolo, T. J. Martínez, S. Lochbrunner, M. Schmitt, J. P. Shaffer, M. Z. Zgierski, and A. Stolow, J. Am. Chem. Soc., 125, 8098/8099 (2003).
K. G. Yager and C. J. Barrett, J. Photochem. Photobiol., C, 182, 250–261 (2006).
J. S. Baskin, L. Bañares, S. Pedersen, and A. H. Zewail, J. Phys. Chem., 100, 11920–11933 (1996).
J. Quenneville and T. J. Martínez, J. Phys. Chem., 107A, 829 (2003).
J. Aihara, Theor. Chem. Acc., 102, 134–138 (1999).
M. Yoshidaa and J. Aihara, Phys. Chem. Chem. Phys., 1, 227–230 (1999).
R. H. El Halabieh, O. Mermut, and C. J. Barrett, Pure Appl. Chem., 76, 1445–1465 (2004).
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © 2015 M. Samadizadeh, S. S. Gorgani.
The text was submitted by the authors in English. Zhurnal Strukturnoi Khimii, Vol. 56, No. 7, pp. 1354-1358, November-December, 2015.
Rights and permissions
About this article
Cite this article
Samadizadeh, M., Gorgani, S.S. Design of a new dihedral-angle-controlled molecular scissors: A DFT investigation. J Struct Chem 56, 1290–1294 (2015). https://doi.org/10.1134/S0022476615070082
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022476615070082