Abstract
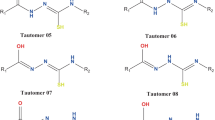
The titled imidazo compound can exist as three tautomers: OH, CH, and NH forms. Firstly, the OH tautomer is produced, which can be tautomerized to the CH and NH tautomers via the intramolecularproton transfer. Herein, employing density functional theory and handling the solvent effects with the PCM model, the structural parameters, energy behavior, and also tautomerization mechanism of the tautomers are investigated. Based on the DFT results and the obtained-AIM parameters, the CH tautomer is considered to be the most stable one. Also, the CH tautomer is a kinetically and thermodynamically controlled product in tautomerization of the OH tautomer in a methanol solution.
Similar content being viewed by others
References
H.-J. Knölker, R. Boese, and R. Hitzemann, Chem. Ber., 123, 327–329 (1990)and references therein.
J. J. Kaminski, J. A. Bristol, C. Puchalski, R. G. Lovey, A. J. Elliott, H. Guzik, D. M. Solomon, D. J. Conn, M. S. Domalski, S. C. Wong, E. H. Gold, J. F. Long, J. S. Chiu, M. Steinberg, and A. T. McPhail, J. Med. Chem., 28, 876–892 (1985).
J. J. Kaminski and A. M. Doweyko, J. Med. Chem., 40, 427–436 (1997).
P. Sanfillipo, M. Urbanski, J. B. Press, B. Dubinsky, and J. B. Moore, J. Med. Chem., 31, 2221–2227 (1988).
A. Gueiffier, M. Lhassani, A. Elhakmaoui, R. Snoeck, G. Andrei, O. Chavignon, J. C. Teulade, A. Kerbal, E. M. Essassi, J. C. Debouzy, M. Witvrouw, Y. Blache, J. Balzarini, E. De Clercq, and J. P. Chapat, J. Med. Chem., 39, 2856–2859 (1996).
A. Gueiffier, S. Mavel, M. Lhassani, A. Elhakmaoui, R. Snoeck, G. Andrei, O. Chavignon, J. C. Teulade, M. Witvrouw, J. Balzarini, E. De Clercq, and J. P. Chapat, J. Med. Chem., 41, 5108–5112 (1998).
M. Lhassani, O. Chavignon, J. M. Chezal, J. C. Teulade, J. P. Chapat, R. Snoeck, G. Andrei, J. Balzarini, E. De Clercq, and A. Gueiffier, Eur. J. Med. Chem., 34, 271–274 (1999).
J. P. Kaplan and P. George, Chem. Abstr., 97, 149531a (1982).
P. George, G. Rossey, M. Sevrin, S. Arbilla, H. Depoortere, and A. E. Wick, in: Imidazopyridines in Anxiety Disorders: A Novel Experimental and Therapeutic Approach, G. Bartholini, M. Garreau, P. L. Morselli, and B. Zivkovic (eds.), Raven Press, New York (1993). pp. 49–59.
J. D. Hoehns and P. J. Perry, Clin. Pharmacol., 12, 814–828 (1993).
B. Edwin and I. H. Joe, J. Mol. Struct., 1034, 119–127 (2013).
H. Eshtiagh-Hosseini, M. R. Housaindokht, S. A. Beyramabadi, S. Beheshti, A. A. Esmaeili, M. Javan-Khoshkholgh, A. Morsali, Spectrochim. Acta, Part A, 71, 1341–1347 (2008).
S. A. Beyramabadi, A. Morsali, M. Javan-Khoshkholgh, and A. A. Esmaeili, Spectrochim. Acta, Part A, 83, 467–471 (2011).
Z. Sadeghzade, S. A. Beyramabadi, and A. Morsali, Spectrochim. Acta, Part A, 138, 637–642 (2015).
S. A. Beyramabadi, A. Morsali, S. H. Vahidi, M. Javan Khoshkholgh, and A. A. Esmaeili, J. Struct. Chem., 53, 665–675 (2012).
H. Eshtiagh-Hosseini, S. A. Beyramabad, A. Morsali, M. Mirzaei, H. Chegini, M. Elahi, and M. A. Naseri, J. Mol. Struct., 1072, 187–194 (2014).
S. H. Vahidi, A. Morsali, and S. A. Beyramabadi, Comput. Theor. Chem., 994, 41–46 (2012).
S. A. Beyramabadi, H. Eshtiagh-Hosseini, M. R. Housaindokht, and A. Morsali, Organometallics, 27, 72–78 (2008).
S. A. Beyramabadi, H. Eshtiagh-Hosseini, M. R. Housaindokht, and A. Morsali, J. Mol. Struct.: THEOCHEM, 903, 108–114 (2009).
H. Eshtiagh-Hosseini, S. A. Beyramabadi, M. R. Housaindokht, and A. Morsali, J. Mol. Struct.: THEOCHEM, 941, 138–143 (2010).
H. Eshtiagh-Hosseini, M. R. Housaindokht, S. A. Beyramabadi, S. H. Mir Tabatabaei, A. A. Esmaeili, and M. Javan- Khoshkholgh, Spectrochim. Acta, Part A, 78, 1046–1050 (2011).
S. A. Beyramabadi, A. Morsali, M. Javan-Khoshkholgh, and A. A. Esmaeili, J. Struct. Chem., 53, 460–467 (2012).
Y. L. Lin and J. Gao, Biochemistry, 49, 84–94 (2010).
W. Rodríguez-Córdoba, J. S. Zugazagoitia, E. Collado-Fregoso, and J. Peon, J. Phys. Chem. A, 111, 6241–6247 (2007).
M. Sauer, C. Yeung, J. H. Chong, B. O. Patrick, and M. J. MacLachlan, J. Org. Chem., 71, 775–788 (2006).
A. Jezierska-Mazzarello, R. Vuilleumier, J. J. Panek, and G. Ciccotti, J. Phys. Chem. B, 114, 242–253 (2010).
M. J. Frisch et al., Gaussian 03, Revision B.03, Gaussian Inc., Pittsburgh, PA (2003).
C. Lee, W. Yang, and R. G. Parr, Phys. Rev. B, 37, 785–789(1988).
R. Cammi and J. Tomasi, J. Comput. Chem., 16, 1449–1458 (1995).
M. Pordel, S. A. Beyramabadi, and A. Mohammadinejad, Dyes Pigm., 102, 46–52 (2014).
X. Li, Y. Wang, S. Zheng, and L. Meng, Struct. Chem., 23, 1233–1240 (2012).
H. Eshtiagh-Hosseini, S. A. Beyramabadi, A. Morsali, M. Mirzaei, H. Chegini, M. Elahi, and M. A. Naseri, J. Mol. Struct., 1072, 187–194 (2014).
P. Gilli, V. Bertolasi, V. Ferretti, and G. Gilli, J. Am. Chem. Soc., 116, 909–915 (1994).
S. Jenkins and I. Morrison, Chem. Phys. Lett., 317, 97–102 (2000).
R. F. Bader, Atoms in Molecules: A Quantum Theory, Oxford University Press, Oxford (1990).
C. F. Matta, A. A. Arabi, and D. F. Weaver, Eur. J. Med. Chem., 45, 1868–1872 (2010).
S. J. Grabowski and M. Malecka, J. Phys. Chem. A, 110, 11847–11854 (2006).
R. N. Musin and Y. H. Mariam, J. Phys. Org. Chem., 19, 425–444 (2006).
X. Fradera, M. A. Austen, and R. F. Bader, J. Phys. Chem. A, 103, 304–314 (1999).
J. Poater, M. Sola, M. Duran, and X. Fradera, Theor. Chem. Acc., 107, 362–371 (2002).
J. G. Angyan, M. Loos, and I. Mayer, J. Phys. Chem., 98, 5244–5248 (1994).
Y. Mo and S. D. Peyerimhoff, J. Chem. Phys., 109, 1687–1697 (1998).
R. F. Bader, M. T. Carroll, J. R. Cheeseman, and C. Chang, J. Am. Chem. Soc., 109, 7968–7979 (1987).
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © 2015 S. A. Beyramabadi, A. Morsali, M. Pordel, H. Chegini, M. Khashi, I. Ahmadi, M. Poorzaki.
The text was submitted by the authors in English. Zhurnal Strukturnoi Khimii, Vol. 56, No. 7, pp. 1318-1326, November-December, 2015.
Rights and permissions
About this article
Cite this article
Beyramabadi, S.A., Morsali, A., Pordel, M. et al. Tautomerization of pyrido[2′,1′:2,3]imidazo[4,5-B]quinoline-12-ylcyanide: A DFT study. J Struct Chem 56, 1253–1261 (2015). https://doi.org/10.1134/S0022476615070045
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022476615070045