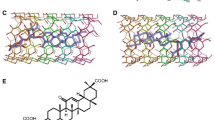
Abstract
The molecular dynamics simulation of dimers of glycyrrhizic acid (GA) arising from the spontaneous meeting of two GA molecules in water is performed. Shown that the molecules in the dimer are quite close to each other, there is no place between them where another molecule (including water molecule) could fit. The relatively stable structures of dimers are found, which are characterized by the specific values of angles between the terpene skeletons of GA molecules and sugar ends. Due to thermal motion, the spontaneous transitions between these structures occur. The insertion of a molecule of cholesterol in the solution showed that the associates formed from two GA molecules and one cholesterol molecule are, as a rule, one of stable GA dimers with the attached cholesterol molecule.
Similar content being viewed by others
References
G. A. Tolstikov, L. A. Boltina, R. M. Kondratenko, et al., Glycyrrhiza: Biodiversity, Chemistry, and Application in Medicine [in Russian], NP “Geo”, Academic Publishing House, Novosibirsk (2007).
T. G. Tolstikova, M. V. Khvostov, and A. O. Bryzgalov, Mini-Rev. Med. Chem., 9, 1317–1328 (2009).
N. E. Polyakov and T. V. Leshina, Open Conf. Proc. J., 2, 64–72 (2011).
V. A. Vavilin, N. F. Salakhutdinov, Yu. I. Ragino, N. E. Polyakov, M. B. Taraban, T. V. Leshina, E. M. Stakhneev, V. V. Lyakhovich, Yu. P. Nikitin, and G. A. Tolstikov, Biomed. Chem., 54, 301–313 (2008).
Yu. I. Ragin, V. A. Vavilin, N. F. Salakhutdinov, S. I. Makarov, E. M. Stakhneva, O. G. Safronova, Yu. P. Nikitin, and G. A. Tolstikov, Bull. Exp. Biol. Med., 145, 285–287 (2008).
N. E. Polyakov, V. K. Khan, and M. B. Taraban, J. Phys. Chem. B, 109, 24526–24530 (2005).
N. E. Polyakov, T. V. Leshina, N. F. Salakhutdinov, et al., J. Phys. Chem. B, 110, 6991–6998 (2006).
N. E. Polyakov, T. V. Leshina, N. F. Salakhutdinov, et al., Free Rad. Biol. Med., 40, 1804–1809 (2006).
N. E. Polyakov, V. K. Khan, M. B. Taraban, et al., J. Phys. Chem. B, 112, 4435–4440 (2008).
N. E. Polyakov, A. Magyar, and L. D. Kispert, J. Phys. Chem. B, 117, No. 35, 10173–10182 (2013).
V. S. Kornievskaya, A. I. Kruppa, N. E. Polyakov, and T. V. Leshina, J. Incl. Phenom. Macrocycl. Chem., 60, 123–130 (2007).
K. C. James and J. B. Stanford, J. Pharm. Pharmacol., 5, 445–450 (1962).
R. J. Gilbert and K. C. James, J. Pharm. Pharmacol., 16, 394–399 (1964).
E. Azaz and R. Segal, Pharm. Acta Helv., 55, 183–186 (1980).
M. Kondo, H. Minamino, and G. Okiyama, J. Soc. Cosmet. Chem., 37, 177–189 (1986).
M. Maskan, J. Food Process Eng., 39, 389–393 (1999).
T. V. Romanenko and Yu. I. Murinov, J. Phys. Chem., 75, 1601–1604 (2001).
O. Yu. Gluschenko, N. E. Polyakov, and T. V. Leshina, Appl. Magn. Res., 41, 283–294 (2011).
T.-T. Chang, M.-F. Sun, K.-C. Chen, et. al., Molec. Simulat., 37, No. 9, 804–811 (2011).
A. V. Lekar, A. A. Milov, S. N. Borisenko, et al., Vestn. Yuzhn. Nauch. Tsentra RAN, 8, No. 2, 18–26 (2012).
D. Van der Spoel, E. Lindahl, B. Hess, G. Groenhof, A. E. Mark, and H. J. C. Berendsen, J. Comput. Chem., 26, No. 16, 1701–1718 (2005).
W. G. Hoover, Phys. Rev. A, 31, 1695–1697 (1985).
M. Parrinello and A. Rahman, J. Appl. Phys., 52, 7182 (1981).
U. Essmann, L. M. Perera, L. Berkowitz, T. A. Darden, H. Lee, and L. G. Pedersen, J. Chem. Phys., 103, 8577–8593 (1995).
A. K. Malde, L. Zuo, M. Breeze, M. Stroet, D. Poger, P. C. Nair, C. Oostenbrink, and A. E. Mark, J. Chem. Theory Comput., 7, No. 12, 4026–4037 (2011).
F. M. Richards, Methods Enzymol., 115, 440–646 (1985).
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © 2015 M. V. Zelikman, A. V. Kim, N. N. Medvedev, O. Yu. Selyutina, N. E. Polyakov.
__________
Translated from Zhurnal Strukturnoi Khimii, Vol. 56, No. 1, pp. 73–82, January–February, 2015.
Rights and permissions
About this article
Cite this article
Zelikman, M.V., Kim, A.V., Medvedev, N.N. et al. Structure of dimers of glycyrrhizic acid in water and their complexes with cholesterol: Molecular dynamics simulation. J Struct Chem 56, 67–76 (2015). https://doi.org/10.1134/S0022476615010102
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022476615010102