Abstract
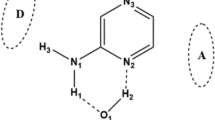
We have demonstrated possibility of proton transfer between nitrogen atom of imidazole ring in histidine and oxygen atom of carboxylic group in aspartic residues inside peptide of Asp-Ala-His+ using density functional theory calculations. Our NBO and AIM analyzes have shown that the proton transfer takes place between side chain of histidine and aspartic acid residues through the hydrogen bond formation. Transition state structures of proton transfer reaction were calculated in gas and solution phases. The calculated reaction rates show that the proton transfer reaction rate in the gas phase is higher than solution phase. The ionization constant (pK a) value of the lysine residue in peptide was estimated to be 1.09 which is lower than intrinsic pK a value of lysine amino acid.
Similar content being viewed by others
References
K. B. Schowen, H. H. Limbach, G. S. Denisov, and R. L. Schowen, Biochim. Biophys. Acta, 1458, No. 1, 43–62 (2000).
G. Schüürmann, M. Cossi, V. Barone, and J. Tomasi, J. Phys. Chem. A, 102, No. 33, 6706 (1998).
H. Li. Jensen, A. D. Robertson, and P. A. Molina, J. Phys.Chem. A, 109, No. 30, 6634–6643 (2005).
D. Roy and J. J. Dannenberg, Chem. Phys. Lett., 512, Nos. 4–6, 255–257 (2011).
J. A. Plumleyand and J. J. Dannenberg, J. Am. Chem. Soc., 132, 1758 (2010).
F. de Brito Mota and R. Rivelino, J. Mol. Struct., 776, Nos. 1–3, 53–59 (2006).
S. Ghosh, S. Mondal, A. Misra, and S. Dalai, J. Mol Struct., 805, Nos. 1–3, 133–141 (2007).
S. Mondal, D. S. Chowdhuri, S. Ghosh, A. Misra, and S. Dalai, J. Mol. Struct., 810, Nos. 1–3, 81–89 (2007).
R. I. Najafabadi, M. R. Housaindokht, M. S. Sadeghi Googheri, M. Sargolzaei, and M. Izadyar, Int. J. Quantum Chem., 112, No. 14, 2675–2680 (2012).
M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, Jr. J. A. Montgomery, T. Vreven, K. N. Kudin, J. C. Burant, J. M. Millam, S. S. Iyengar, J. Tomasi, V. Barone, B. Mennucci, M. Cossi, G. Scalmani, N. Rega, G. A. Petersson, H. Nakatsuji, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, M. Klene, X. Li, J. E. Knox, H. P. Hratchian, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, P. Y. Ayala, K. Morokuma, G. A. Voth, P. Salvador, J. J. Dannenberg, V. G. Zakrzewski, S. Dapprich, A. D. Daniels, M. C. Strain, O. Farkas, D. K. Malick, A. D. Rabuck, K. Raghavachari, J. B. Foresman, J. V. Ortiz, Q. Cui, A. G. Baboul, S. Clifford, J. Cioslowski, B. B. Stefanov, G. Liu, A. Liashenko, P. Piskorz, I. Komaromi, R. L. Martin, D. J. Fox, T. Keith, M. A. Al-Laham, C. Y. Peng, A. Nanayakkara, M. Challacombe, P. M. W. Gill, B. Johnson, W. Chen, M. W. Wong, C. Gonzalez, and J. A. Pople, Gaussian, Inc., Wallingford CT (2004).
A. D. Becke, J. Chem. Phys., 98, 5648–5652 (1993).
R. G. Parr and W. Yang, Density-Functional Theory of Atoms and Molecules, Oxford University Press, New York, (1989).
S. Miertuš and E. T. Scrocco, J. Chem. Phys., 55, 117–129 (1981).
P. Hudáky and A. Perczel, J. Phys. Chem. A, 108, No. 29, 6195–6205 (2004).
H. Eyring, J. Chem. Phys., 3, 107–115 (1935).
E. Espinosa, E. Molins, and C. Lecomte, Chem. Phys. Lett., 285, 170–173 (1998).
A. E. Reed, L. A. Curtiss, and F. Weinhold, Chem. Rev., 88, No. 6, 899–926 (1988).
R. F. W. Bader, Atoms in Molecules: A. Quantum Theory, Oxford University Press, Oxford, UK (1990).
R. Bader, Atoms in Molecules: A Quantum Theory, Oxford University Press, Oxford, USA (1994).
R. F. W. Bader, Chem. Rev., 91, 893–928 (1991).
R. F. W. Bader, Acc. Chem. Res., 18, 9–15 (1985).
Y. Nozaki and C. Tanford, Methods Enzymol., 11, 715–734 (1967).
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © 2014 M. Sargolzaei, M. Afshar, M.S. Sadeghi, H. Hamidian.
The text was submitted by the authors in English.
Rights and permissions
About this article
Cite this article
Sargolzaei, M., Afshar, M., Sadeghi, M. et al. First principles study on proton transfer between amino acid side chains of histidine and aspartic acid in β-structure. J Struct Chem 55, 1627–1634 (2014). https://doi.org/10.1134/S0022476614080332
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022476614080332