Abstract
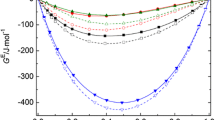
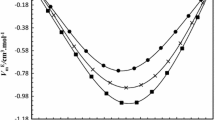
Kirkwood-Buff integrals are calculated from the thermodynamic data for binary mixtures of water with methanol, ethanol, 1-propanol, and 2-propanol at a temperature of 298.15 K in the pressure range from atmospheric to 100 MPa. The values of local compositions Δn ij are calculated which characterize the excess (or deficit) of molecules i around the central molecule j. It is found that the pressure affects destructively the homoassociation in all mixtures studied. In a series MeOH < EtOH < 2-PrOH < 1-PrOH an excess of molecules around the similar type molecules increases in the local environment and the pressure effect on the local composition is enhanced.
Similar content being viewed by others
References
J. G. Kirkwood and F. P. Buff, J. Chem. Phys., 19, No. 6, 774 (1951).
E. Ruckenstein and I. Shulgin, J. Phys. Chem. B, 104, No. 11, 2540 (2000).
E. A. Ploetz and P. E. Smith, J. Chem. Phys., 135, No. 4, 044506-1 (2011).
M. C. A. Donkersloot, J. Solution Chem., 8, No. 4, 293 (1979).
E. Matteoli and L. Lepori, J. Chem. Phys., 80, No. 6, 2856 (1984).
I. Shulgin and E. Ruckenstein, J. Phys. Chem. B, 103, No. 13, 2496 (1999).
Y. Marcus, Monatsch. Chem., 132, No. 11, 1387 (2001).
A. Perera, F. Sokolić, L. Almásy, and Y. Koga, J. Chem. Phys., 110, No. 25, 12707 (2006).
A. Ben-Naim, A. M. Navarro, and J. M. Leal, Phys. Chem. Chem. Phys., 10, No. 18, 2451 (2008).
L. Lepori and E. Matteoli, J. Phys. Chem., 92, No. 24, 6997 (1988).
A. Ben-Naim, J. Phys. Chem., 93, No. 9, 3809 (1989).
A. Ben-Naim, Pure Appl. Chem., 62, 25 (1990).
A. M. Zaichikov and M. A. Krest’yaninov, J. Struct. Chem., 50, No. 4, 647–656 (2009).
E. Matteoli and L. Lepori, J. Chem. Soc. Faraday Trans., 91, No. 3, 431 (1995).
E. Matteoli, J. Phys. Chem. B, 101, No. 47, 9800 (1997).
I. L. Shulgin and E. Ruckenstein, J. Phys. Chem. B, 110, No. 25, 12707 (2006).
I. L. Shulgin and E. Ruckenstein, Phys. Chem. Chem. Phys., 10, No. 8, 1097 (2008).
J. Hu, C. A. Haynes, A. H. Y. Wu, C. M. W. Cheung, M. M. Chen, E. G. M. Yee, T. Ichioka, K. Nishikawa, P. Westh, and Y. Koga, Can. J. Chem., 81, No. 2, 141 (2003).
G. C. Benson and O. Kiyohara, J. Solution Chem., 9, No. 10, 791 (1980).
A. J. Easteal and L. A. Woolf, J. Chem. Thermodyn., 17, No. 1, 69 (1985).
A. J. Easteal and L. A. Woolf, J. Chem. Thermodyn., 17, No. 1, 49 (1985).
D. Pečar and V. Doleček, Fluid Phase Equil., 230, Nos. 1/2, 36 (2005).
E. Matteoli and L. Lepori, J. Mol. Liq., 47, Nos. 1—3, 89 (1990).
Y. Miyamotoa, M. Takemoto, M. Hosokawa, Y. Uosaki, and T. Moriyoshi, J. Chem. Thermodyn., 22, No. 10, 1007 (1990).
G. I. Egorov, V. N. Afanas’ev, and A. M. Kolker, Zh. Obshch. Khim., 74, No. 2, 194 (2004).
G. I. Egorov and D. M. Makarov, Zh. Fiz. Khim., 82, No. 6, 1175 (2008).
A. L. Zaitsev, V. E. Petrenko, and Y. M. Kessler, J. Solution Chem., 18, No. 2, 115 (1989).
L. Dougan, S. P. Bates, R. Hargreaves, J. P. Fox, J. Crain, J. L. Finney, V. Réat, and A. K. Soper, J. Chem. Phys., 121, No. 13, 6456 (2004).
A. Perera, L. Zoranić, F. Sokolić, and R. Mazighi, J. Mol. Liq., 159, No. 1, 52 (2011).
Y. Marcus, Phys. Chem. Chem. Phys., 1, 2975 (1999).
H. Hayashi, K. Nishikawa, and T. Iijima, J. Phys. Chem., 94, No. 21, 8334 (1990).
K. Yoshida and T. Yamaguchi, Z. Naturforsch., 56a, 529 (2001).
A. B. Roney, B. Space, E. W. Castner, R. L. Napoleon, and P. B. Moore, J. Phys. Chem. B, 108, No. 22, 7389 (2004).
E. V. Ivanov and V. K. Abrosimov, J. Struct. Chem., 46, No. 5, 856–861 (2005).
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © 2014 D. M. Makarov, G. I. Egorov.
__________
Translated from Zhurnal Strukturnoi Khimii, Vol. 55, No. 2, pp. 283–289, March–April, 2014.
Rights and permissions
About this article
Cite this article
Makarov, D.M., Egorov, G.I. Analysis of the pressure effect on the local composition in a water-alkanol mixture using Kirkwood-Buff integrals. J Struct Chem 55, 263–269 (2014). https://doi.org/10.1134/S0022476614020103
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022476614020103