Abstract
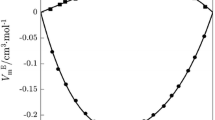
The structural and thermodynamic properties are calculated for mixtures of aprotic amides with water and acetonitrile. The simulation approach is used to identify the specific and nonspecific components of the total energy of intermolecular interactions, which are used to calculate the corresponding contributions to the enthalpy of mixing. The negative enthalpies of mixing in the aqueous mixtures are found to be caused not by heterocomponent specific interactions, but by nonspecific ones. The difference in the structural and thermodynamic properties of the aqueous and nonaqueous mixtures of aprotic amides is shown to be largely due to the behavior of the hydrogen bond network of water and the packing of the resulting solutions.
Similar content being viewed by others
References
G. Hradetzky, I. Hammerl, W. Kisan, K. Wehner, and H. J. Bittrich, Data of Selective Solvents, Verlag der Wissenschaften, Berlin (1989).
V. N. Kartsev, M. N. Rodnikova, J. Bartel, et al., Zh. Fiz. Khim., 76, No. 6, 1016 (2002).
M. R. J. Dack, Chem. Soc. Rev., 4, No. 1, 211 (1975).
Yu. M. Kessler and A. L. Zaitsev, Solvophobic Effects [in Russian], Khimiya, Leningrad (1989).
V. N. Kartsev, M. N. Rodnikova, and S. N. Shtykov, J. Struct Chem., 45, No. 1, 96–99 (2004).
A. M. Zaichikov, J. Struct Chem., 53, No. 3, 476 (2012).
A. M. Zaichikov and M. A. Krest’yaninov, J. Struct Chem., 49, No. 2, 285–295 (2008).
A. M. Zaichikov, J. Struct Chem., 47,Suppl., P. S73–S80 (2006).
A. M. Zaichikov and M. A. Krest’yaninov, J. Struct Chem., 50, No. 4, 647–656 (2009).
H. Piekarski, K. Kubalczyk, and M. Wasiak, J. Chem. Eng. Data, 55, No. 12, 5435 (2010).
Yu. G. Bushuev and V. P. Korolev, in: Concentrated and Saturated Solutions [in Russian], A. M. Kutepov (ed.), Nauka, Moscow (2003), pp. 255–313.
W. Xie, J. Pu, A. D. MacKerell, et al., J. Chem. Theory Comput., 3, No. 6, 1878 (2007).
A. M. Zaichikov and A. G. Krestov, Zh. Fiz. Khim., 69, No. 3, 389 (1995).
A. M. Zaichikov and Yu. G. Bushuev, Zh. Fiz. Khim., 69, No. 11, 1942 (1995).
A. M. Zaichikov and M. A. Krest’yaninov, J. Struct Chem., 49, No. 4, 688–695 (2008).
S. Miyanaga, K. Tamura, and S. Murakami, J. Therm. Anal., 38, No. 12, 1767 (1992).
A. M. Zaichikov, Y. G. Bushuev, and G. A. Krestov, J. Therm. Anal., 45, No. 4, 687 (1995).
F. Moučka and I. Nezbeda, J. Mol. Liq., 159, No. 1, 47 (2011).
E. B. Bagley, T. P. Nelson, and J. M. Scigliano, J. Phys. Chem., 77, No. 23, 2794 (1973).
M. Costas, S. N. Bhattacharyya, and D. Patterson, J. Chem. Soc., Faraday Trans. I, 81, No. 1, 387 (1985).
H. T. French, J. Chem. Thermod., 21, No. 8, 801 (1989).
G. I. Egorov, E. L. Gruznov, and A. M. Kolker, Zh. Fiz. Khim., 70, No. 2, 216 (1996).
R. Gomes de Azevedo, J. Szydlowski, P. F. Pires, et al., J. Chem. Thermod., 36, 211 (2004).
V. P. Belousov and M. Yu. Panov, Thermodynamics of Aqueous Nonelectrolyte Solutions [in Russian], Khimiya, Leningrad (1983).
A. Panuszko, E. Gojło, J. Zielkiewicz, et al., J. Phys. Chem. B, 112, No. 8, 2483 (2008).
E. S. Balankina and A. K. Lyashchenko, J. Mol. Liq., 103/104, No. 1, 211 (2003).
T. Radnai, S. Itoh, and H. Ohtaki, Bull. Chem. Soc. Jpn., 61, No. 18, 3845 (1988).
V. I. Savelłev, Extended Abstract of Cand. Sci. (Chem.) Dissertation, Ivanovo State University of Chemistry and Technology, Ivanovo (2000).
Z. V. Pachuliya, Extended Abstract of Cand. Sci. (Chem.) Dissertation, Ivanovo Institute of Chemistry and Technology, Ivanovo (1985).
D. Ben-Amotz, F. O. Raineri, and G. Stell, J. Phys. Chem. B, 109, No. 14, 6866 (2005).
B. Lee, J. Phys. Chem., 87, No. 1, 112 (1983).
A. Ben-Naim, J. Solut. Chem., 30, No. 5, 475 (2001).
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © 2013 A. M. Zaichikov, M. A. Krest’yaninov.
__________
Translated from Zhurnal Strukturnoi Khimii, Vol. 54, Supplement 2, pp. S341–S349, 2013.
Rights and permissions
About this article
Cite this article
Zaichikov, A.M., Krest’yaninov, M.A. Structural and thermodynamic properties and intermolecular interactions in aqueous and acetonitrile solutions of aprotic amides. J Struct Chem 54 (Suppl 2), 336–344 (2013). https://doi.org/10.1134/S0022476613080131
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022476613080131