Abstract
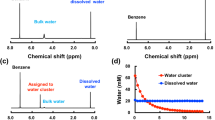
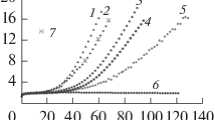
In the work the character of water clusterization in the whole existence domain of its liquid state is discussed: from supercooled states to the critical point. Conclusions about the cluster composition of liquid water are drawn based on the analysis: 1) of the features of dielectric relaxation; 2) character of the temperature dependence of its static dielectric permittivity, and 3) the value and temperature dependence of different contributions to the heat capacity of the system. It is shown that near the water crystallization point tetramers prevail in its structure, with an increase in the temperature trimers start to play the main role, and near the critical point of water dimers become the major associates. At temperatures near the water crystallization point the obtained results well agree with the data on emission and absorption X-ray spectroscopy.
Similar content being viewed by others
References
Y. Zhao and D. G. Truhlar, J. Phys. Chem., A110, 5121 (2006).
D. Eisenberg and W. Kauzmann, The Structure and Properties of Water, Oxford University, New York (1969).
A. Mogelhoy, A. Kelkhanen, and K. T. Wikfeldt, J. Phys. Chem. B, 115, 14149 (2011).
V. L. Kulinskii, N. P. Malomuzh, and O. I. Matvejchuk, Physica A, 388, 4560 (2009).
A. Nilsson and L. G. M. Petterson, Chem. Phys., 389, 1 (2011).
J.-H. Guo, J. Luo, and A. Augustsson, Phys. Rev. Lett., 89, 137402 (2002).
P. Vernet and D. Nordlund, Science, 304, 995 (2004).
S. V. Lishchuk, N. P. Malomuzh, and P. V. Makhlaichuk, Phys. Lett. A, 375, 2656 (2011).
Ya. I. Frenkel, Kinetic Theory of Liquids [in Russian], Nauka, Leningrad (1975).
A. Rahman, Phys. Rev. A, 136, 405 (1964).
C. A. Kroxton, Liquid State Physics — A Statistical Mechanical Introduction, Cambridge University Press, Cambridge (1974).
L. A. Bulavin, T. V. Lokotosh, and N. P. Malomuzh, J. Mol. Liq., 137, 1 (2008).
T. V. Lokotosh, N. P. Malomuzh, and K. N. Pankratov, J. Chem. Eng. Data, 55, 2021 (2010).
L. A. Bulavin, A. I. Fisenko, and N. P. Malomuzh, Chem. Phys. Lett., 453, 183 (2008).
L. A. Bulavin, V. L. Kulinskii, and N. P. Malomuzh, J. Mol. Liq., 161, 19 (2011).
A. I. Fisenko and N. P. Malomuzh, Chem. Phys., 345, 164 (2008).
A. I. Fisenko and N. P. Malomuzh, Int. J. Mol. Sci., 10, 2383 (2009).
K. Okada, M. Yao, Y. Hiejima, H. Kohno, and Y. Kojihara, J. Chem. Phys., 110, 3026 (1999).
H. R. Pruppacher, J. Chem. Phys., 56, 101 (1972).
K. Simpson and M. Karr, Phys. Rev., 17, 342 (1958).
K. A. Valiev and E. N. Ivanov, Usp. Fiz. Nauk, 109, 31 (1973).
R. C. West (ed.), CRS Handbook of Chemistry and Physics: a Ready-Reference Book of Chemical and Physical Data, 67th ed, CRS Press, Boca Raton (1996).
J. Teixeira, M.-C. Bellissent-Funel, S.-H. Chen, and J. Dianoux, Phys. Rev., A31, 1913 (1985).
P. Blanckenhagen, Ber. Bunsenges. Phys. Chem., 76, 891 (1972).
S. Magazu, F. Migliardo, and M. T. F. Telling, J. Phys. Chem. B, 110, 1020 (2006).
P. Wernet, D. Nordlund, U. Bergmann, M. Cavalleri, M. Odelius, H. Ogasawara, L. A. Naslund, T. K. Hirsch, L. Ojamae, P. Glatzel, L. G. M. Pettersson, and A. Nilsson, Science, 304, 995 (2004).
H. Fröhlich,, Theory of Dielectrics, Oxford University Press, London (1958).
D. P. Fernandez, Y. Mulev, A. R. H. Goodwin, et al., J. Phys. Chem. Ref. Data, 24, No. 1, 33–69 (1995).
M. Uematsy and E. U. Frank, J. Phys. Chem. Ref. Data, 9, No. 4, 1291–1306 (1980).
A. D. Chistyakov, Zh. Fiz. Khim., 81, No. 1, 11 (2007).
V. N. Makhlaichik and S. V. Khrapatyi, Zh. Fiz. Khim., (2013), (to be published).
N. K. Frank, J. D. Cruzan, and R. J. Saykally, Chem. Rev., 103, 2533–2577 (2003).
J. K. Gregory, D. C. Clary, K. Liu, M. G. Brown, and R. J. Saykally, Science, 275, 814 (1997).
J. B. Paul, C. P. Collier, R. J. Saykally, J. J. Scherer, and A. O’Keefe, J. Phys. Chem. A, 101, 5211–5214 (1997).
V. L. Kulinskii and N. P. Malomuzh, in: Soft Matter under Exogenic Impacts. NATO Science Series II, J. Rzoska and V. A. Mazur (eds.), 242, 287–301 (2007).
L. Wang, J. Zhao, and H. Fang, J. Phys. Chem. C, 112, 11779–11785 (2008).
F. H. Stillinger and C. W. David, J.Chem. Phys., 69, 1473 (1978).
Jongseob Kim, Seung Bum Suh, and Kwang S. Kim, J. Chem. Phys., 111, 10077 (1999).
C. N. Ramachandran and N. Sathyamurthy, Chem. Phys. Lett., 410, 348–351 (2005).
L. Wang et al., J. Phys. Chem. C, 112, No. 31, 16417–16421 (2008).
D. J. Wales and M. P. Hodgesr, Chem. Phys. Lett., 286, 65–72 (1998).
P. V. Makhlaichik, M. P. Malomuzh, and I. V. Zhuganiuk, Ukrain. J. Phys., 58, No. 3, 278–288 (2013).
P. V. Makhlaichik, M. P. Malomuzh, and I. V. Zhuganiuk, Ukrain. J. Phys., 58. (to be published) (2013).
A. Oleinikova, I. Brovchenko, N. Smolin, A. Krukau, A. Geiger, and R. Winter, Phys. Rev. Lett., 95, 247802 (2005).
L. B. Partay, P. Jedlovszky, I. Brovchenko, and A. Oleinikova, Phys. Chem. Chem. Phys., 9, 1341–1346 (2007).
L. Pártay and P. Jedlovszky, J. Chem. Phys., 123. 024502 (2005).
K. M. Benjamin, A. J. Schultz, and D. A. Kofke, Ind. Eng. Chem. Res., 45, 5566–5573 (2006).
N. Goldman, R. S. Fellers, C. Leforestier, and R. J. Saykally, J. Phys. Chem. A, 105, No. 3, 515–519 (2001).
Y. Scribano, N. Goldman, R. J. Saykally, and C. Leforestier, J. Phys. Chem. A, 110, 5411–5419 (2006).
J. Hargrove, Chem. Phys. Discuss., 7, 11123–11140 (2007).
V. I. Serdyukov, L. N. Sinitsa, and Yu. A. Poplavskii, Pis’ma v Zh. Teor. i Èksp. Fiz., 89, No. 1, 12–15 (2009).
I. A. Gorchakova, G. V. Chlenova, and A. A. Vigasin, Optika Atmosfery i Okeana, 22, No. 6, 546–551 (2009).
A. H. Harvey and E. W. Lemmon J. Phys. Chem. Ref. Data, 33, 369 (2004).
G. T. Evans and V. Vaida, J. Chem. Phys., 113, 6652 (2000).
L. A. Curtiss, D. J. Frurip, and M. Blander, J. Chem. Phys., 71(6), 2703–2711 (1979).
A. A. Vigasin, A. I. Pavlyuchko, Y. Jin, and S. Ikawa, J. Mol. Struct., 742, 173–181 (2005).
L. D. Landau and E. M. Lifshits, Statistical Physics [in Russian], Part 1, Nauka, Moscow (1976).
Ya. I. Frenkel, Kinetic Theory of Liquids. [in Russian], Nauka, Leningrad (1975).
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © 2013 N. P. Malomuzh, V. N. Makhlaichuk, P. V. Makhlaichuk, K. N. Pankratov.
__________
Translated from Zhurnal Strukturnoi Khimii, Vol. 54, Supplement 2, pp. S210–S225, 2013.
Rights and permissions
About this article
Cite this article
Malomuzh, N.P., Makhlaichuk, V.N., Makhlaichuk, P.V. et al. Cluster structure of water in accordance with the data on dielectric permittivity and heat capacity. J Struct Chem 54 (Suppl 2), 205–220 (2013). https://doi.org/10.1134/S0022476613080039
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022476613080039