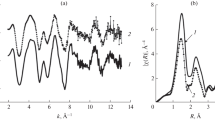
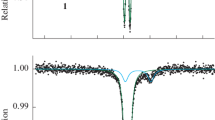
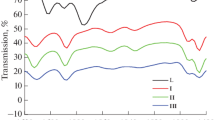
Abstract
EPR and Mössbauer spectroscopy is used to study a new liquid crystal complex of iron(III) with a Schiff base: 4,4′-dodecyloxybenzoyloxybenzoyl-4-oxysalicylidene-2-aminopyridine with a PF −6 counterion. It is shown that Fe(III) ions exist only in the high-spin (HS, S = 5/2) state. It is found that under the influence of temperature the system demonstrates the stepwise behavior of the product of the integrated intensity of EPR lines (I) and temperature (proportional to χ T, where χ is the magnetic susceptibility) with an inflection point at ∼80 K. Above 80 K a new EPR spectrum is detected due to the excited S = 2 state and the formation of dimeric molecules (through oxygen bridges) with a strong intramolecular antiferromagnetic exchange interaction J 1 = 162.1 cm−1. Below 80 K iron(III) complexes are organized in 1D chains where the exchange value J 2 = 2.1 cm−1. At 80 K there is a structural phase transition in the system: the transition from a 1D chain organization of HS Fe(III) centers to dimeric molecules. Based on quantum chemical calculations a model of the binuclear iron(III) complex is proposed.
Similar content being viewed by others
References
P. Gütlich and H. A. Goodwin (eds.), in: Spin Crossover in Transition Metal Compounds I, Topics in Current Chemistry, v. 233, Springer, Heidelberg (2004).
S. Hayami, Z. Gu, M. Shiro, Y. Einaga, A. Fujishima, O. Sato, J. Am. Chem. Soc., 122, 7126/7127 (2000).
E. Pretsch, P. Bühlmann, and C. Affolter, Structure Determination of Organic Compounds, Tables of Spectral Data, Springer (2000).
H. Becker, R. Beckert, W. Berger, et al., Organikum, v. 1, Berlin (2008).
A. A. Saleh, A. M. Tawfik, S. M. Abu-El-wafa, and H. F. Osman, J. Coord. Chem., 57, No. 14, 1191–1204 (2004).
S. Chandra and U. Kumar, Spectrochim. Acta, Part A, 61, Nos. 1/2, 219–224 (2005).
T. M. A. Ismail, J. Coord. Chem., 59, No. 3, 255–270 (2006).
J. P. Perdew, K. Burke, and M. Ernzerhof, Phys. Rev. Lett., 77, 3865–3868 (1996)
J. P. Perdew, K. Burke, and M. Ernzerhof, Phys. Rev. Lett., 78, 1396 (1997).
A. D. Becke, J. Chem. Phys., 98, 5648–5652 (1993)
P. J. Stephens, F. J. Devlin, C. F. Chabalowski, and M. J. Frisch, J. Phys. Chem., 98, 11623–11627 (1994).
D. N. Laikov, PRIRODA, Electronic Structure Code, Version 5 (2005).
M. J. Frisch, G. W. Trucks, H. B. Schlegel, et al., GAUSSIAN 09, Revision A.02, Gaussian, Inc., Wallingford, CT (2009).
F. Neese, ORCA — an Ab Initio, Density Functional and Semiempirical Program Package, 2.8, University of Bonn, Bonn, Germany (2011).
D. N. Laikov, Development of the Economical Approach to the Calculation of Molecules by the Density Functional Theory Method and Its Application to the Solution of Complex Chemical Problems [in Russian], Diss. ... Cand. of Phys.-Math. Sc., Moscow State University (2000).
A. Schafer, H. Horn, and R. Ahlrichs, J. Chem. Phys., 100, 5829–5835 (1994).
L. Noodleman and J. G. Norman, J. Chem. Phys., 70, 4903–4906 (1979)
L. Noodleman, J. Chem. Phys., 74, 5737–5743 (1981)
L. Noodleman and E. R. Davidson, Chem. Phys., 109, 131–143 (1986)
L. Noodleman and D. A. Case, Adv. Inorg. Chem., 38, 423–470 (1992).
E. Ruiz, J. Cano, S. Alvarez and P. Alemany, J. Comp. Chem., 20, 1391–1400 (1999)
E. Ruiz, A. Rodriguez- Fortea, J. Cano, S. Alvarez, and P. Alemany, J. Comp. Chem., 24, 982–989 (2003)
E Ruiz., S. Alvarez, J. Cano, and V. Polo, J. Chem. Phys., 123, 164110 (2005).
E. Ruiz, P. Alemany, S. Alvarez and J. Cano, J. Am. Chem. Soc., 119, 1297–1303 (1997)
E. Ruiz, J. Cano, S. Alvarez and P. Alemany, J. Am. Chem. Soc., 120, 11122–11129 (1998)
E. Ruiz, J. Cano, S. Alvarez, A. Caneschi, and D. Gatteschi, J. Am. Chem. Soc., 125, 6791–6794 (2003)
P. Ghosh, E. Bill, T. Weyhermuller, F. Neese, and K. Wieghardt, J. Am. Chem. Soc., 125, 1293–1308 (2003)
J. Cano, R. Costa, S. Alvarez, and E. Ruiz, J. Chem. Theory and Comput., 3, 782–788 (2007)
E. M. Zueva, M. M. Petrova, R. Herchel, Z. Trávníček, R. Raptis, L. Mathivathanan, and J. E. McGrady, Dalton Trans., 30, 5924–5932 (2009).
H. H. Wickman, M. P. Klein, and D. A. Shirley, J. Chem. Phys., 42, No. 6, 2113–2117 (1965).
R. Aasa, J. Chem. Phys., 52, No. 8, 3919–3930 (1970).
B. Weber, C. Carbonera, C. Desplances, and J-F. Letard, Eur. J. Inorg. Chem., 10, 1589–1598 (2008).
A. Bousseksou, J. Nasser, J. Linares, K. Boukheddaden, and F. Varret, J. Phys. I, France, 2, 1381–1403 (1992).
H. Spiering, T. Kohlhaas, H. Romstedt, A. Hauser, C. Bruns-Yilmaz, J. Kusz, and P. Gutlich, Coord. Chem. Rev., 190–192, 629–647 (1999).
B. Weber and E.-G. Jager, Eur. J. Inorg. Chem., 4, 465–477 (2009).
H. Weihe and H. U. Güdel, J. Am. Chem. Soc., 119, 6539–6543 (1997).
C. J. O’Connor, Progr. Inorg. Chem., 29, 203–283 (2007).
D. Collison and A. K. Powell, Inorg. Chem., 29, 4735–4746 (1990).
R. Dingle, M. E. Lines, and S. L. Holt, Phys. Rev., 187, 643–648 (1969).
R. L. Carlin, R. Burriel, J. A. Rojo, and F. Palacio, Inorg. Chem., 23, No. 15, 2213–2215 (1984).
N. Bouslimani, N. Clement, G. Rogez, P. Turek, M. Bernard, S. Dagorne, D. Martel, H. N. Cong, and R. Welter, Inorg. Chem., 47, 7623–7630 (2008).
J. Owen, J. Appl. Phys., 32, 213S–217S (1961).
M. Y. Okamura and B. M. Hoffman, J. Chem. Phys., 51, 3128/3129 (1969).
A. Ozarowski, B. R. McGarvey, and J. E. Drake, Inorg. Chem., 34, No. 22, 5558–5566 (1995).
H. Wickman, M. P. Klein, and D. A. Shirley, Phys. Rev., 152, No. 1, 345–357 (1966).
A. I. Aleksanderov, T. V. Pashkova, D. V. Barakhtenko, M. S. Gruzdev, and U. V. Chervonova, Zhidkie Kristally, 4, No. 38, 14–22 (2011).
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text Copyright © 2013 by N. E. Domracheva, V. E. Vorob’eva, A. V. Pyataev, R. A. Manapov, E. M. Zueva, M. S. Gruzdev, U. V. Chervonova
__________
Translated from Zhurnal Strukturnoi Khimii, Vol. 54, Supplement 1, pp. S20–S31, 2013.
Rights and permissions
About this article
Cite this article
Domracheva, N.E., Vorob’eva, V.E., Pyataev, A.V. et al. Stepwise magnetic behavior of the liquid crystal iron(III) complex. J Struct Chem 54 (Suppl 1), 16–27 (2013). https://doi.org/10.1134/S0022476613070020
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022476613070020