Abstract
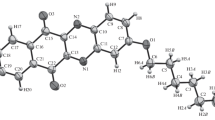
Phthalazinylhydrazone of 2-formylpyrrole is synthesized, the values of ionization constants are determined, and quantum chemical calculation of the geometry and total energy of possible tautomers is performed. The structure of the cyclic oxidation product of hydrazone 3-(1H-pyrrolyl-2)-[1,2,4]-triazolo(3,4-a)phthalazine existing in the crystal in the form of dimers linked by two hydrogen bonds is described.
Similar content being viewed by others
References
K. N. Zelenin, L. A. Khorseeva, and V. V. Alekseev, Pharm. Chem. J., 26, No. 5, 395 (1992).
P. Vicini, M. Incerty, I. A. Doytchinova, et al., Eur. J. Med. Chem., 48, No. 5, 624 (2006).
B. Segura-Pacheco, C. Trejo-Becerril, E. Perez-Cardenas, et al., Clin. Cancer Res., 9, No. 5, 1596 (2003).
L. M. Kaminskas, S. M. Pyke, and P. C. Burcham, J. Pharm. Exper. Therap., 310, No. 3, 1003 (2004).
L. M. Kaminskas, S. M. Pyke, and P. C. Burcham, Org. Biomol. Chem., 18, No. 2, 2578 (2004).
H. J. Knowles, Y. M. Tian, D. R. Mole, and A. L. Harris, Circ. Res., 95, No. 2, 162 (2004).
P. A. Reece, Med. Res. Rev., 1, No. 1, 73 (1981).
V. A. Kogan, S. I. Levchenkov, L. D. Popov, and I. N. Shcherbakov, Russ. J. Gen. Chem., 79, No. 1, 2767 (2009).
K. Nakashima, K. Shimada, and S. Akiyama, Chem. Pharm. Bull., 33, No. 4, 1515 (1985).
T. Razvi, M. Ramalingam, and P. B. Sattur, Ind. J. Chem. Sect. B, 28, No. 8, 987 (1989).
G. Giorgi, F. Ponticelli, L. Chiasserini, and C. Pellerano, J. Chem. Soc., Perkin Trans. 2, No. 11, 2259 (2000).
T. Odashima, M. Yamada, N. Yonemori, and H. Ishi, Bull. Chem. Soc. Jpn., 60, No. 9, 3225 (1987).
L. D. Popov, I. N. Shcherbakov, S.I. Levchenkov, et al., J. Coord. Chem., 61, No. 3, 392 (2008).
L. D. Popov, I. N. Shcherbakov, S. I. Levchenkov, et al., Russ. J. Coord. Chem., 37, No. 7, 483 (2011).
L. D. Popov, I. N. Shcherbakov, S. I. Levchenkov, et al. Russ. J. Gen. Chem., 80, No. 12, 2501 (2010).
L. D. Popov, I. N. Shcherbakov, S. I. Levchenkov, et al., Russ. J. Gen. Chem., 82, No. 3, 465 (2012).
Bruker APEX2 Software Package, Bruker AXS, Madison (2005).
G. M. Sheldrik, SADABS, Program for Scanning and Correction of Area Detector Data, Univ. Göttingen, Germany (2004).
G. M. Sheldrick, Acta Crystallogr. A, 64, No. 1, 112 (2008).
M. J. Frisch, G. W. Trucks, H. B. Schlegel, et al., Gaussian 03, Revision D.01, Gaussian, Inc., Wallingford CT (2004).
P. J. Stephens, F. J. Devlin, C. F. Chabalowski, and M. J. Frisch, J. Phys. Chem., 98, No. 45, 11623 (1994).
A. D. Becke, J. Chem. Phys., 98, No. 7, 5648 (1993).
C. Lee, W. Yang, and R. G. Parr, Phys. Rev. B, 37, No. 2, 785 (1988).
G. A. Zhurko and D. A. Zhurko, Chemcraft Version 1.6 (build 338): http://www.chemcraftprog.com.
B. I. Buzykin, N. N. Bystrykh, A. P. Stolyarov, et al., Khim. Geterotsikl. Soedin., 14, No. 3, 575 (1978).
R. J. Butcher, J. P. Jasinski, H. S. Yathirajan, et al., Acta Crystallogr., E63, No. 9, o3674 (2007).
O. Büyükgüngör, M. Odabasoglu, A. M. Vijesh, and H. S. Yathirajan, Acta Crystallogr., E63, No. 10, o4084 (2007)
M. A. Bryleva, A. N. Kravtsova, I. N. Shcherbakov, et al., J. Struct. Chem., 53, No. 2, 295 (2012).
A. A. Shoukry and M. M. Shoukry, Spectrochim. Acta. A, 70, No. 3, 686 (2008).
H. Zimmer and A. Amer, Heterocycles, 26, No. 5, 1177 (1987).
F. H. Allen, O. Kennard, D. G. Watson, et al., J. Chem Soc., Perkin Trans. 2., No. 12, S1 (1987).
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text Copyright © 2013 by L. D. Popov, S. I. Levchenkov, I. N. Shcherbakov, G. G. Aleksandrov, Yu. P. Tupolova, V. V. Lukov, O. I. Askalepova, V. A. Kogan
__________
Translated from Zhurnal Strukturnoi Khimii, Vol. 54, No. 3, pp. 565–569, May–June, 2013.
Rights and permissions
About this article
Cite this article
Popov, L.D., Levchenkov, S.I., Shcherbakov, I.N. et al. Crystal structure of the polycyclic oxidation product of 1′-phthalazinylhydrazone of 2-formylpyrrole. J Struct Chem 54, 619–623 (2013). https://doi.org/10.1134/S0022476613030232
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022476613030232