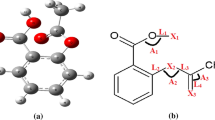
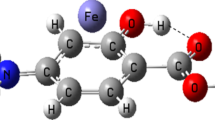
Abstract
A systematic computational study was carried out to determine the structural stability of salicylic acid derivatives as well as the acidic properties of the protonation-deprotonation site and excitation parameters of the considered drugs at different temperatures using quantum chemical calculations. For further structural information, the dipole moment and differences in the energy of the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) as Δ(HOMO-LUMO) were compared. A high stability of the salicylic acid derivatives was referred to the large HOMO-LUMO band gap. The result of the current study may give useful information about the drug biochemical functionality based on the physical and chemical nature at different temperatures, and in this way the study on the structural features of analogous molecules of salicylic acid derivatives with higher drug functionality would be possible.
Similar content being viewed by others
References
A. G. Gilman, J. G. Hardman, and L. E. Limbird, The Pharmacological Basis of Therapeutics, 10th ed, McGraw Hill, New York (2001).
Y. Sasada, T. Takano, and M. Kakudo, Bull. Chem. Soc. Jpn., 37, 940–946 (1964).
L. L. Zamora, J. M. Calatayud, J. M. Caiatayud, and Y. F. Mestre, Anal. Chim. Acta, 394, 159–163 (1999).
F. Pertlik, Monatsh. Chem., 121, 129–139 (1990).
K. W. J. Street and G. H. Schenk, J. Pharm. Sci., 70, 641–645 (1981).
M. I. Walash, A. M. E. Brashy, and M. A. Sultan, Mikrochim. Acta, 113, 113–124 (1994).
A. R. Medina, M. L. F. Cordova, and A. M. Diaz, Anal. Chim. Acta, 394, 149–158 (1999).
M. J. M. Takac and D. V. Topic, Europ. J. Pharm. Sci., 17, S47–S57 (2002).
X. Xu and K. S. Pang, J. Pharmacokinetics and Pharmacodynamics, 17(6), 645–671 (1989).
D. H. Litina, Current Med. Chem., 7, 375–388 (2000).
R. G. Parr and W. Yang, Density Functional Theory of Atoms and Molecules, Oxford Univ Press, Oxford (1989).
M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, J. A. Montgomery Jr., T. Vreven, K. N. Kudin, J. C. Burant, J. M. Millam, S. S. Iyengar, J. Tomasi, V. Barone, B. Mennucci, M. Cossi, G. Scalmani, N. Rega, G. A. Petersson, H. Nakatsuji, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, M. Klene, X. Li, J. E. Knox, H. P. Hratchian, J. B. Cross, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, P. Y. Ayala, K. Morokuma, G. A. Voth, P. Salvador, J. J. Dannenberg, V. G. Zakrzewski, S. Dapprich, A. D. Daniels, M. C. Strain, O. Farkas, D. K. Malick, A. D. Rabuck, K. Raghavachari, J. B. Foresman, J. V. Ortiz, Q. Cui, A. G. Baboul, S. Clifford, J. Cioslowski, B. B. Stefanov, G. Liu, A. Liashenko, P. Piskorz, I. Komaromi, R. L. Martin, D. J. Fox, T. Keith, M. A. Al-Laham, C. Y. Peng, A. Nanayakkara, M. Challacombe, P. M. W. Gill, B. Johnson, W. Chen, M. W. Wong, C. Gonzalez, and J. A. Pople, Gaussian Inc., Pittsburgh PA (1998).
M. Kato, K. Nihikawa, M. Untani, M. Mlyazaki, M. Takcmura, and J. Bmehem, Gaussian 98, Revision A.7, 107, 242–247 (1990).
M. Braäuer, M. Kunert, E. Dinjus, M. Klußmann, M. Doring, H. Corls, and E. Anders, J. Mol. Struct. (Theochem.), 505, 298–301 (2000).
C. Lu, Y. L. Chung, and K. F. Chang, J. Hazardous Materials B, 138, 304–310 (2006).
V. G. Maltarollo, P. H. Mello, and K. M. Honorio, J. Molecular Modeling, 16, No. 4, 799–804.
A. M. Teixeira, J. Walkimar, and M. Carneiro, J. Molecular Structure: Theochem., Issues 1–3, 30 May, 335, 255–266 (1995).
K. Nakatani, T. Matsuno, K. Adachi, S. Hagihara, and I. Saito, J. Am. Chem. Soc., 20, 123, No. 24, 695–702 (2001).
G. Balogh, E. Csizer, G. F. Gyorgy, Z. Halmos, B. Herenyi, P. Horvath, A. Lauko, and G. Sandor, (1995) Pharmaceutical Research, 12, 2295–2298 (2000).
J. V. Aukunuru, U. B. Kompella, and G. V. Betageri, J. Liquid Chromatography & Related Technologies, 23, No. 4, 565–578 (2000).
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text Copyright © 2012 by E. Mousavinezhad Sarasia, M. E. S. Soliman, B. Honarparvar
The text was submitted by the authors in English. Zhurnal Strukturnoi Khimii, Vol. 53, No. 3, pp. 580–587, May–June, 2012.
Rights and permissions
About this article
Cite this article
Mousavinezhad Sarasia, E., Soliman, M.E.S. & Honarparvar, B. Theoretical study on the molecular electronic properties of salicylic acid derivatives as anti- inflammatory drugs. J Struct Chem 53, 574–581 (2012). https://doi.org/10.1134/S0022476612030237
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022476612030237