Abstract
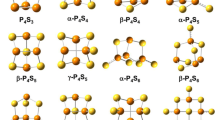
The structure and thermodynamics of the following phosphorus oxide caged clusters were calculated in the gas phase at STP via both the local density approximation (LDA) and a generalized gradient approximation (BLYP) of density functional theory: the experimentally characterized trioxide (P4O6) and pentoxide (P4O10), and in order of thermodynamic preference, the hypothetical P24O60, P8O20, P24O48, and P20O20. All of the hypothetical oxides would dissociate to the pentoxide at equilibrium. Secondarily, the LDA calculation of the enthalpy of formation was unexpectedly superior to the BLYP calculation.
Similar content being viewed by others
References
F. J. Owens, J. Mol. Struct. Theochem., 623, 197 (2003)
S. Evangelisti, J. Phys. Chem. A, 102, 4925 (1998)
L. Gagliardi, G. Orlandi, S. Evangelisti, and B. O. Roos, J. Chem. Phys., 114, 10733 (2001).
M. Gausa, R. Kaschner, G. Seifert, et al., J. Chem. Phys., 104, 9719 (1996).
M. Ystenes, W. Brockner, and F. Menzel, Vib. Spectr., 5, 195 (1993)
R. O. Jones and G. Seifert, J. Chem. Phys., 96, 2942 (1992)
L. Operti, G. A. Vaglio, M. Peruzzini, and P. Stoppioni, Inorg. Chim. Acta, 96, 43 (1985).
A. Pfitzner, S. Reiser, and T. Nilges, Angew. Chem. Int. Ed., 39, 4160 (2000).
F. A. Cotton and G. Wilkinson, Advanced Inorganic Chemistry, 5th Ed., John Wiley, New York (1988), Ch. 11.
B. T. Sterenberg, L. Scoles, and A. J. Carty, Coord. Chem. Rev., 231, 183 (2002).
T. M. Klapötke, Angew. Chem. Int. Ed., 42, 3461 (2003).
A. Tellenbach and M. Jansen, Angew. Chem. Int. Ed., 40, 4691 (2001).
M. J. Moses, J. C. Fettinger, and B. W. Eichhorn, Science, 300, 778 (2003).
A. V. Bulgakov, O. F. Bobrenok, I. Ozerov, et al., Appl. Phys. A, 79, 1369 (2004)
A. V. Bulgakov, O. F. Bobrenok, and V. I. Kosyakov, Chem. Phys. Lett., 320, 19 (2000).
A. V. Bulgakov, O. F. Bobrenok, V. I. Kosyakov, et al., Phys. Solid State, 44, 617 (2002).
O. Sedo, Z. Voràc, M. Alberti, and J. Havel, Polyhedron, 23, 1199 (2004).
M. Häser, U. Schnieder, and R. Ahlrichs, J. Am. Chem. Soc., 114, 9551 (1992).
C.-H. Hu, M. Shen, and H. F. Schaefer, Theor. Chim. Acta, 88, 29 (1994).
M. Shen and F. Schaefer III, J. Chem. Phys., 101, 2261 (994 )
T. Baruah, M. R. Pederson, R. R. Zope, and M. R. Beltrn, Chem. Phys. Lett., 387, 476 (2004).
K. Raghavachari, R. C. Haddon, and J. S. Binkley, Chem. Phys. Lett., 122, 219 (1985)
R. O. Jones and D. Hohl, J. Chem. Phys., 92, 6710 (1990)
R. O. Jones and G. Seifert, J. Chem. Phys., 96, 7564 (1992)
H. Zhang and K. Balasubramanian, J. Chem. Phys., 97, 3437 (1992)
K. A. Peterson, R. A. Kendall, and T. A. Dunning, J. Chem. Phys., 99, 9790 (1993)
P. Ballone and R. O. Jones, J. Chem. Phys., 100, 4941 (1994)
N. R. Brinkmann, G. S. Tschumper, and H. F. Schaefer, J. Chem. Phys., 110, 6240 (1999)
B. Song and P. Cao, Phys. Lett. A, 291, 343 (2001)
S. S. Wesolowski, N. R. Brinkmann, E. F. Valeev, et al., J. Chem. Phys., 116, 112 (2002)
P. Ballone and R. O. Jones, J. Chem. Phys., 121, 8147 (2004)
L. Guo, H. C. Wu, and Z. H. Jin, J. Mol. Struct.-Theochem., 677, 59 (2004).
L. Rulisek, Z. Havlas, S. Hermanek, and J. Plesek, Can. J. Chem., 76, 1274 (1998).
G. Seifert, T. Heine, and P. W. Fowler, Eur. Phys. J. D, 16, 341 (2001).
R. C. Mowrey, B. A. Williams, and C. H. Douglass, J. Phys. Chem. A, 101, 5748 (1997).
Y. Moussaoui, O. Ouamerali, and G. R. De Maré, Int. Rev. Phys. Chem., 22, 641 (2003).
N. L. Haworth, G. B. Bacskay, and J. C. Mackie, J. Phys. Chem. A, 106, 1533 (2002).
Y. Wang, J. Xu, Z. Cao, and Q. Zhang, J. Phys. Chem. B, 108, 4579 (2004).
R. A. LaViolette and M. T. Benson, J. Chem. Phys., 112, 9296 (2000).
J. D. Cox, D. D. Wagman, and V. A. Medvedev, CODATA Key Values for Thermodynamics; Hemisphere Publishing, New York (1989), Also online at http://webbook.nist.gov/chemistry/
B. J. Delley, Chem. Phys., 92, 508 (1990)
B. J. Delley, Chem. Phys., 113, 7756 (2000).
J. P. Perdew and Y. Wang, Phys. Rev. B, 45, 13244 (1992).
A. D. Becke, J. Chem. Phys., 88, 2547 (1988).
C. Lee, W. Yang, and R. G. Parr, Phys. Rev. B, 37, 785 (1988).
S. J. Vosko, L. Wilk, and M. Nusair, Can. J. Phys., 58, 1200 (1980).
J. Ochterski, Thermochemistry in Gaussian, Gaussian, Inc.: Pittsburgh (2002), Also Online at http://www.gaussian.com/g whitepap/thermo.htm
J. Cioslowski, G. Liu, and P. Piskorz, J. Phys. Chem. A, 102, 9890 (1998).
T. Hirano, in: MOPAC 7.0, J. J. P. Stewart (ed.), Indiana U.: Bloomington (1993).
H. Bock and H. Müller, Inorg. Chem., 23, 4365 (1984).
J. R. Fincke, R. P. Anderson, T. Hyde, et al., Plasma Chem. Plasma Process, 22, 105 (2002)
R. A. LaViolette, R. Berry, and R. McGraw, Plasma Chem. Plasma Process, 16, 249 (1996).
B. Beagley, D. W. J. Cruickshank, T. G. Hewitt, and A. Haaland, Trans. Faraday Soc., 63, 836 (1967).
B. Beagley, D. W. J. Cruickshank, T. G. Hewitt, and K. H. Jost, Trans. Faraday Soc., 65, 1219 (1969).
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text Copyright © 2012 by R. A. LaViolette and M. T. Benson
The text was submitted by the authors in English. Zhurnal Strukturnoi Khimii, Vol. 53, No. 1, pp. 54–59, January–February, 2012.
Rights and permissions
About this article
Cite this article
LaViolette, R.A., Benson, M.T. Structure and thermodynamics of phosphorus oxide caged clusters. J Struct Chem 53, 48–54 (2012). https://doi.org/10.1134/S0022476612010064
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022476612010064