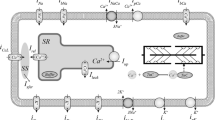
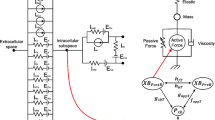
Abstract
A complex sequence of electrical and mechanical processes at different levels of the heart organization provides its pumping function. The force generation by the contractile proteins of cardiomyocytes requires energy expenditure. In addition, some ion transport mechanisms in the cell consume ATP to redistribute ions between various cell structures and transfer them to the extracellular space during the excitation-contraction of the cardiomyocyte. Intracellular mitochondria are involved in ATP synthesis. We have developed an integrative mathematical model of a human left ventricular cardiomyocyte, which describes the processes of electromechanical coupling in the cell, the process of ATP synthesis in mitochondria in the tricarboxylic acid cycle, and the utilization of ATP by the activities of various pathways. The integrative model shows that a change in the mechanical conditions of cardiomyocyte contraction in the normal state (for example, a change in its initial length or applied load) not only affects the time course of the action potential in the cell but also modulates the processes associated with the ATP production and utilization. The mechano-dependence of mitochondrial energetics is provided by a complex system of mechano-electric, mechano-calcium and mechano-metabolic direct links and feedbacks. Our simulations reveal that despite the mechano-dependent changes, the ATP level remains within the range of values characteristic of healthy myocardium.







Similar content being viewed by others
REFERENCES
Orini M, Nanda A, Yates M, Di Salvo C, Roberts N, Lambiase PD, Taggart P (2017) Mechano-electrical feedback in the clinical setting: Current perspectives. Prog Biophys Mol Biol 130: 365–375. https://doi.org/10.1016/j.pbiomolbio.2017.06.001
Markhasin VS, Solov’eva O, Chumarnaia TV, Sukhareva SV (2009) The problem of myocardial heterogeneity. Ross Fiziol Zh Im I M Sechenova 95: 919–943.
Solovyova O, Katsnelson LB, Kohl P, Panfilov AV, Tsaturyan AK, Tsyvian PB (2016) Mechano-electric heterogeneity of the myocardium as a paradigm of its function. Prog Biophys Mol Biol 120: 249–254. https://doi.org/10.1016/j.pbiomolbio.2015.12.007
Cortassa S, Aon MA, O’Rourke B, Jacques R, Tseng H-J, Marbán E, Winslow RL (2006) A computational model integrating electrophysiology, contraction, and mitochondrial bioenergetics in the ventricular myocyte. Biophys J 91: 1564–1589. https://doi.org/10.1529/biophysj.105.076174
Brandes R, Bers DM (1999) Analysis of the mechanisms of mitochondrial NADH regulation in cardiac trabeculae. Biophys J 77: 1666–1682. https://doi.org/10.1016/S0006-3495(99)77014-1
Tinker A, Aziz Q, Thomas A (2014) The role of ATP-sensitive potassium channels in cellular function and protection in the cardiovascular system. Br J Pharmacol 171: 12–23. https://doi.org/10.1111/bph.12407
Bazhutina A, Balakina-Vikulova NA, Kursanov A, Solovyova O, Panfilov A, Katsnelson LB (2021) Mathematical modelling of the mechano-electric coupling in the human cardiomyocyte electrically connected with fibroblasts. Prog Biophys Mol Biol 159: 46–57. https://doi.org/10.1016/j.pbiomolbio.2020.08.003
ten Tusscher KH, Panfilov AV (2006) Alternans and spiral breakup in a human ventricular tissue model. Am J Physiol Heart Circ Physiol 291: H1088–H1100. https://doi.org/10.1152/ajpheart.00109.2006
Sulman T, Katsnelson LB, Solovyova O, Markhasin VS (2008) Mathematical modeling of mechanically modulated rhythm disturbances in homogeneous and heterogeneous myocardium with attenuated activity of Na+–K+ pump. Bull Math Biol 70: 910–49. https://doi.org/10.1007/s11538-007-9285-y
Jafri MS, Rice JJ, Winslow RL (1998) Cardiac Ca2+ dynamics: the roles of ryanodine receptor adaptation and sarcoplasmic reticulum load. Biophys J 74(3): 1149–1168. https://doi.org/10.1016/S0006-3495(98)77832-4
Rice JJ, Jafri MS, Winslow RL (2000) Modeling short-term interval-force relations in cardiac muscle. Am J Physiol Heart Circ Physiol 278: H913–H931. https://doi.org/10.1152/ajpheart.2000.278.3.H913
Balakina-Vikulova NA, Panfilov A, Solovyova O, Katsnelson LB (2020) Mechano-calcium and mechano-electric feedbacks in the human cardiomyocyte analyzed in a mathematical model. J Physiol Sci 70: 12. https://doi.org/10.1186/s12576-020-00741-6
Regnier M, Martyn DA, Chase PB (1998) Calcium regulation of tension redevelopment kinetics with 2-deoxy-ATP or low [ATP] in rabbit skeletal muscle. Biophys J 74: 2005–2015. https://doi.org/10.1016/S0006-3495(98)77907-X
Rice JJ, Winslow RL, Hunter WC (1999) Comparison of putative cooperative mechanisms in cardiac muscle: length dependence and dynamic responses. Am J Physiol 276: H1734–1754. https://doi.org/10.1152/ajpheart.1999.276.5.H1734
Vahl C, Timek T, Bonz A, Fuchs H, Dillman R, Hagl S (1998) Length dependence of calcium-and force-transients in normal and failing human myocardium. J Mol Cell Cardiol 30: 957–966. https://doi.org/10.1006/jmcc.1998.0670
Pieske B, Sutterlin M, Schmidt-Schweda S, Minami K, Meyer M, Olschewski M, Holubarsch C, Just H, Hasenfuss G (1996) Diminished post-rest potentiation of contractile force in human dilated cardiomyopathy. Functional evidence for alterations in intracellular Ca2+ handling. J Clin Invest 98: 764–776. https://doi.org/10.1172/JCI118849
Brixius K, Hoischen S, Reuter H, Lasek K, Schwinger RH (2001) Force/shortening-frequency relationship in multicellular muscle strips and single cardiomyocytes of human failing and nonfailing hearts. J Card Fail 7: 335–341. https://doi.org/10.1054/jcaf.2001.29902
Yabe T, Mitsunami K, Inubushi T, Kinoshita M (1995) Quantitative Measurements of Cardiac Phosphorus Metabolites in Coronary Artery Disease by 31P Magnetic Resonance Spectroscopy. Circulation 92: 15–23. https://doi.org/10.1161/01.CIR.92.1.15
Page G, Ratchada P, Miron Y, Steiner G, Ghetti A, Miller PE, Reynolds JA, Wang K, Greiter-Wilke A, Polonchuk L, Traebert M, Gintant GA, Abi-Gerges N (2016) Human ex-vivo action potential model for pro-arrhythmia risk assessment. J Pharmacol Toxicol Methods 81: 183–195. https://doi.org/10.1016/j.vascn.2016.05.016
Vahl CF, Timek T, Bonz A, Kochsiek N, Fuchs H, Schaffer L, Rosenberg M, Dillmann R, Hagl S (1997) Myocardial length-force relationship in end stage dilated cardiomyopathy and normal human myocardium: analysis of intact and skinned left ventricular trabeculae obtained during 11 heart transplantations. Basic Res Cardiol 92: 261–270. https://doi.org/10.1007/BF00788521
Izakov V, Katsnelson LB, Blyakhman FA, Markhasin VS, Shklyar TF (1991) Cooperative effects due to calcium binding by troponin and their consequences for contraction and relaxation of cardiac muscle under various conditions of mechanical loading. Circ Res 69: 1171–1184. https://doi.org/10.1161/01.RES.69.5.1171
Yasuda S, Sugiura S, Yamashita H, Nishimura S, Saeki Y, Momomura S, Katoh K, Nagai R, Sugi H (2003) Unloaded shortening increases peak of Ca2+ transients but accelerates their decay in rat single cardiac myocytes. Am J Physiol Heart Circ Physiol 285: H470–475. https://doi.org/10.1152/ajpheart.00012.2003
Hatano A, Okada J, Washio T, Hisada T, Sugiura S (2011) A three-dimensional simulation model of cardiomyocyte integrating excitation-contraction coupling and metabolism. Biophys J 101: 2601–2610. https://doi.org/10.1016/j.bpj.2011.10.020
Youm JB, Choi SW, Jang CH, Kim HK, Leem CH, Kim N, Han J (2011) A computational model of cytosolic and mitochondrial [Сa2+] in paced rat ventricular myocytes. Korean J Physiol Pharmacol 15: 217–239. https://doi.org/10.4196/kjpp.2011.15.4.217
Matsuoka S, Sarai N, Jo H, Noma A (2004) Simulation of ATP metabolism in cardiac excitation–contraction coupling. Prog Biophys Mol Biol 85: 279–299. https://doi.org/10.1016/j.pbiomolbio.2004.01.006
Cortassa S, Aon MA, Marban E, Winslow RL, O’Rourke B (2003) An integrated model of cardiac mitochondrial energy metabolism and calcium dynamics. Biophys J 84: 2734–2755. https://doi.org/10.1016/S0006-3495(03)75079-6
Saks V, Dzeja P, Schlattner U, Vendelin M, Terzic A, Wallimann T (2006) Cardiac system bioenergetics: metabolic basis of the Frank-Starling law. J Physiol 571: 253–273. https://doi.org/10.1113/jphysiol.2005.101444
ten Tusscher KH, Noble D, Noble PJ, Panfilov AV (2004) A model for human ventricular tissue. Am J Physiol Heart Circ Physiol 286: H1573–1589. https://doi.org/10.1152/ajpheart.00794.2003
Solovyova O, Vikulova N, Katsnelson LB, Markhasin VS, Noble P, Garny A, Kohl P, Noble D (2003) Mechanical interaction of heterogeneous cardiac muscle segments in silico: effects on Ca2+ handling and action potential. Int J Bifurcat Chaos 13: 3757–3782. https://doi.org/10.1142/S0218127403008983
Solovyova O, Vikulova N, Markhasin V, Kohl P (2003) A novel method for quantifying the contribution of different intracellular mechanisms to mechanically induced changes in action potential characteristics. LNCS 2674: 8–17. https://doi.org/10.1007/3-540-44883-7_2
Peyronnet R, Nerbonne JM, Kohl P (2016) Cardiac Mechano-Gated Ion Channels and Arrhythmias. Circ Res 118: 311–329. https://doi.org/10.1161/CIRCRESAHA.115.305043
Boycott HE, Nguyen MN, Vrellaku B, Gehmlich K, Robinson P (2020) Nitric Oxide and Mechano-Electrical Transduction in Cardiomyocytes. Front Physiol 11: 606740. https://doi.org/10.3389/fphys.2020.606740
Ch’en FF, Vaughan-Jones RD, Clarke K, Noble D (1998) Modelling myocardial ischaemia and reperfusion. Prog Biophys Mol Biol 69: 515–538. https://doi.org/10.1016/S0079-6107(98)00023-6
Crampin EJ, Smith NP, Langham AE, Clayton RH, Orchard CH (2006) Acidosis in models of cardiac ventricular myocytes. Philos Trans R Soc Lond A 364: 1171–1186. https://doi.org/10.1098/rsta.2006.1763
Rodriguez B, Ferrero JM Jr, Trenor B (2002) Mechanistic investigation of extracellular K+ accumulation during acute myocardial ischemia: a simulation study. Am J Physiol Heart Circ Physiol 283: H490–H500. https://doi.org/10.1152/ajpheart.00625.2001
Loewe A, Wülfers EM, Seemann G (2018) Cardiac ischemia—insights from computational models. Herzschrittmacherther Elektrophysiol 29: 48–56. https://doi.org/10.1007/s00399-017-0539-6
Michailova A, Saucerman J, Belik ME, McCulloch AD (2005) Modeling regulation of cardiac KATP and L-type Ca2+ currents by ATP, ADP, and Mg2+. Biophys J 88: 2234–2249. https://doi.org/10.1529/biophysj.104.046284
Vikulova NA, Vasilyeva AD, Zamaraev DE, Solovyova OE, Markhasin VS (2014) Modeling of disturbances in electrical and mechanical function of cardiomyocytes under acute ischemia. Biophysics 59: 791–799. https://doi.org/10.1134/s0006350914050297
Weiss JN, Venkatesh N, Lamp ST (1992) ATP‐sensitive K+ channels and cellular K+ loss in hypoxic and ischaemic mammalian ventricle. The Journal of Physiology 447: 649–673. https://doi.org/10.1113/jphysiol.1992.sp019022
Kagiyama Y, Hill JL, Gettes LS (1982) Interaction of acidosis and increased extracellular potassium on action potential characteristics and conduction in guinea pig ventricular muscle. Circ Res 51: 614–623. https://doi.org/10.1161/01.RES.51.5.614
Irisawa H, Sato R (1986) Intra- and extracellular actions of proton on the calcium current of isolated guinea pig ventricular cells. Circ Res 59: 348–355. https://doi.org/10.1161/01.res.59.3.348
Noma A (1983) ATP-regulated K+ channels in cardiac muscle. Nature 305: 147–148. https://doi.org/10.1038/305147a0
Funding
The study was supported by the Russian Science Foundation grant no. 21-14-00226.
Author information
Authors and Affiliations
Contributions
Conceptualization and study design, data analysis, manuscript preparation—N.A.B.-V. and L.B.K.; models integration, writing software, performing numerical experiments, processing the results obtained—N.A.B.-V.
Corresponding authors
Ethics declarations
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest.
Additional information
Translated by A. Polyanovsky
Supplementary Information
The online version contains supplementary material available at https://doi.org/10.1134/S0022093022070122.
Rights and permissions
About this article
Cite this article
Balakina-Vikulova, N.A., Katsnelson, L.B. Integrative Mathematical Model of Electrical, Metabolic and Mechanical Processes in Human Cardiomyocytes. J Evol Biochem Phys 58 (Suppl 1), S107–S124 (2022). https://doi.org/10.1134/S0022093022070122
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022093022070122