Abstract
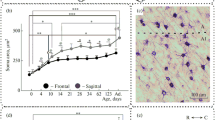
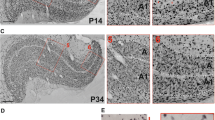
The postnatal developmental dynamics of neurons in the cat dorsal lateral geniculate nucleus (dLGN) was studied using a selective marker of Y neurons, the monoclonal antibody SMI-32 against non-phosphorylated heavy-chain neurofilaments. The neuronal soma area was measured within various functional regions of the nucleus. The following three major peculiarities of the internal organization of the dLGN layers and retinotopic regions were revealed: (1) an ascending dorsoventral soma area gradient of SMI-32-immunopositive neurons, increasing with age; (2) a descending centroperipheral soma area gradient of SMI-32-immunopositive neurons, decreasing with age; (3) smaller size of neurons located in the lower visual field representation along the vertical meridian. The data suggest heterogeneity of the population of Y neurons and heterochronicity of its postnatal development.




Similar content being viewed by others
REFERENCES
Brodmann K (1909) Vergleichende Lokalisationslehre der Grosshirnirinde in ihren Prinzipien dargestelt auf Grund des Zellenbaues. Barth JA, Leipzig.
Al Ghamdi KS, Polgár E, Todd AJ (2009) Soma size distinguishes projection neurons from neurokinin 1 receptor-expressing interneurons in lamina I of the rat lumbar spinal dorsal horn. Neuroscience 164: 1794–1804. https://doi.org/10.1016/j.neuroscience.2009.09.071
Beebe NL, Young JW, Mellott JG, Schofield BR (2016) Extracellular molecular markers and soma size of inhibitory neurons: evidence for four subtypes of GABAergic cells in the inferior colliculus. J Neurosci 36: 3988–3999. https://doi.org/10.1523/JNEUROSCI.0217-16.2016
Lingley AJ, Bowdridge JC, Farivar R, Duffy KR (2018) Mapping of neuron soma size as an effective approach to delineate differences between neural populations. J Neurosci Methods 304: 126–135. https://doi.org/10.1016/j.jneumeth.2018.04.018
Davis MR, Fernald RD (1990) Social control of neuronal soma size. J Neurobiol 21: 1180–1188. https://doi.org/10.1002/neu.480210804
Rabinowicz T, Petetot JM-C, Khoury JC, de Courten-Myers GM (2009) Neocortical maturation during adolescence: Change in neuronal soma dimension. Brain Cogn 69: 328–336. https://doi.org/10.1016/j.bandc.2008.08.005
Payne BR, Peters A (2002) The Concept of Cat Primary Visual Cortex. In: Payne BR, Peters A (eds) The Cat Primary Visual Cortex. Elsevier, San Diego; London; Boston; New York; Sydney; Tokyo; Toronto, pp 1–129.
Bowling DB, Caverhill JI (1989) ON/OFF organization in the cat lateral geniculate nucleus: Sublaminae vs. columns. J Comp Neurol 283: 161–168. https://doi.org/10.1002/cne.902830114
Murphy PC, Duckett SG, Sillito AM (2000) Comparison of the laminar distribution of input from areas 17 and 18 of the visual cortex to the lateral geniculate nucleus of the cat. J Neurosci 20: 845–853. https://doi.org/10.1523/JNEUROSCI.20-02-00845.2000
Bowling DB, Wieniawa-Narkiewicz E (1986) The distribution of on- and off-centre X- and Y-like cells in the A layers of the cat’s lateral geniculate nucleus. J Physiol 375: 561–572. https://doi.org/10.1113/jphysiol.1986.sp016133
Westland KW, Burke W (2002) Patterns of X and Y optic nerve fibre terminations in the dorsal lateral geniculate nucleus of the cat. Doc Ophthalmol 105: 129–149. https://doi.org/10.1023/A:1020544802517
Mastronarde DN (1987) Two classes of single-input X-cells in cat lateral geniculate nucleus. I. Receptive-field properties and classification of cells. J Neurophysiol 57: 357–380. https://doi.org/10.1152/jn.1987.57.2.357
Enroth-Cugell C, Robson JG (1984) Functional characteristics and diversity of cat retinal ganglion cells. Basic characteristics and quantitative description. Invest Ophthalmol Vis Sci 25: 250–267.
Saul AB (2008) Lagged cells. NeuroSignals 16: 209–225. https://doi.org/10.1159/000111564
Bickford ME, Guido W, Godwin DW (1998) Neurofilament proteins in Y-cells of the cat lateral geniculate nucleus: Normal expression and alteration with visual deprivation. J Neurosci 18: 6549–6557. https://doi.org/10.1523/JNEUROSCI.18-16-06549.1998
Duffy KR, Crowder NA, LeDue EE (2012) Investigation of cytoskeleton proteins in neurons of the cat lateral geniculate nucleus. J Comp Neurol 520: 186–199. https://doi.org/10.1002/cne.22727
Hockfield S, Sur M (1990) Monoclonal antibody Cat-301 identifies Y-cells in the dorsal lateral geniculate nucleus of the cat. J Comp Neurol 300: 320–330. https://doi.org/10.1002/cne.903000305
Mikhalkin A, Nikitina N, Merkulyeva N (2021) Heterochrony of postnatal accumulation of nonphosphorylated heavy-chain neurofilament by neurons of the cat dorsal lateral geniculate nucleus. J Comp Neurol 529: 1430–1441. https://doi.org/10.1002/cne.25028
Merkulyeva N, Mikhalkin A, Zykin P (2018) Early postnatal development of the lamination in the lateral geniculate nucleus A-layers in cats. Cell Mol Neurobiol 38: 1137–1143. https://doi.org/10.1007/s10571-018-0585-6
Merkulyeva NS, Veshchitskii A, Makarov F, Gerasimenko Y, Musienko P (2016) Distribution of 28 kDa calbindin-immunopositive neurons in the cat spinal cord. Front Neuroanat 9: 166. https://doi.org/10.3389/fnana.2015.00166
Mikhalkin AA, Merkulyeva NS (2021) Peculiarities of Age-Related Dynamics of Neurons in the Cat Lateral Geniculate Nucleus as Revealed in Frontal versus Sagittal Slices. J Evol Biochem Physiol 57: 1001–1007. https://doi.org/10.1134/S0022093021050021
Sternberger LA, Sternberger NH (1983) Monoclonal antibodies distinguish phosphorylated and nonphosphorylated forms of neurofilaments in situ. Proc Natl Acad Sci U S A 80: 6126–6130. https://doi.org/10.1073/pnas.80.19.6126
Nurzynska K, Mikhalkin A, Piorkowski A (2017) CAS: cell annotation software—research on neuronal tissue has never been so transparent. Neuroinformatics 15: 365–382. https://doi.org/10.1007/s12021-017-9340-2
Quené H, Van Den Bergh H (2004) On multi-level modeling of data from repeated measures designs: A tutorial. Speech Commun 43: 103–121. https://doi.org/10.1016/j.specom.2004.02.004
Kutcher MR, Duffy KR (2007) Cytoskeleton alteration correlates with gross structural plasticity in the cat lateral geniculate nucleus. Vis Neurosci 24: 775–785. https://doi.org/10.1017/S095252380707068X
Van Der Gucht E, Vandesande F, Arckens L (2001) Neurofilament protein: A selective marker for the architectonic parcellation of the visual cortex in adult cat brain. J Comp Neurol 441: 345–368. https://doi.org/10.1002/cne.1416
Kalil R (1978) Development of the dorsal lateral geniculate nucleus in the cat. J Comp Neurol 182: 265–291. https://doi.org/10.1002/cne.901820206
Hickey TL (1980) Development of the dorsal lateral geniculate nucleus in normal and visually deprived cats. J Comp Neurol 189: 467–481. https://doi.org/10.1002/cne.901890304
Carden WB, Guido W, Ziburkus J, Datskovskaia A, Godwin DW, Bickford ME (2000) A novel means of Y cell identification in the developing lateral geniculate nucleus of the cat. Neurosci Lett 295: 5–8. https://doi.org/10.1016/S0304-3940(00)01581-0
Ho KC, Gwozdz JT, Hause LL, Antuono PG (1989) Correlation of neuronal cell body size in motor cortex and hippocampus with body height and body weight. J Neuropathol Exp Neurol 48: 361. https://doi.org/10.1097/00005072-198905000-00188
Coleman LA, Friedlander MJ (2002) Postnatal dendritic development of Y‐like geniculocortical relay neurons. Int J Dev Neurosci 20: 137–159. https://doi.org/10.1016/S0736-5748(02)00018-7
Humphrey AL, Sur M, Uhlrich DJ, Sherman SM (1985) Projection patterns of individual X- and Y-cell axons from the lateral geniculate nucleus to cortical area 17 in the cat. J Comp Neurol 233: 159–89. https://doi.org/10.1002/cne.902330203
Ling C, Hendrickson ML, Kalil RE (2012) Morphology, classification, and distribution of the projection neurons in the dorsal lateral geniculate nucleus of the rat. PLoS One 7: e49161. https://doi.org/10.1371/journal.pone.0049161
Hoffmann K-P, Sireteanu R (1977) Interlaminar differences in the effects of early and late monocular deprivation on the visual acuity of cells in the lateral geniculate nucleus of the cat. Neurosci Lett 5: 171–175. https://doi.org/10.1016/0304-3940(77)90042-8
Yeh C-I, Stoelzel CR, Alonso J-M (2003) Two different types of Y cells in the cat lateral geniculate nucleus. J Neurophysiol 90: 1852–1864. https://doi.org/10.1152/jn.00417.2003
Lee D, Lee C, Malpeli JG (1992) Acuity-sensitivity trade-offs of X and Y cells in the cat lateral geniculate complex: role of the medial interlaminar nucleus in scotopic vision. J Neurophysiol 68: 1235–1247. https://doi.org/10.1152/jn.1992.68.4.1235
Frascella J, Lehmkuhle S (1984) A comparison between Y-cells in A-laminae and lamina C of cat dorsal lateral geniculate nucleus. J Neurophysiol 52: 911–920. https://doi.org/10.1152/jn.1984.52.5.911
Yeh CI, Stoelzel CR, Weng C, Alonso JM (2009) Functional consequences of neuronal divergence within the retinogeniculate pathway. J Neurophysiol 101: 2166–2185. https://doi.org/10.1152/jn.91088.2008
Tootle JS, Friedlander MJ (1986) Postnatal development of receptive field surround inhibition in kitten dorsal lateral geniculate nucleus. J Neurophysiol 56: 523–541. https://doi.org/10.1152/jn.1986.56.2.523
Van Horn SC, Erişir A, Sherman SM (2000) Relative distribution of synapses in the A-laminae of the lateral geniculate nucleus of the cat. J Comp Neurol 416: 509–520. https://doi.org/10.1002/(SICI)1096-9861(20000124)416:4<509::AID-CNE7>3.0.CO;2-H
Duffy KR, Fong MF, Mitchell DE, Bear MF (2018) Recovery from the anatomical effects of long-term monocular deprivation in cat lateral geniculate nucleus. J Comp Neurol 526: 310–323. https://doi.org/10.1002/cne.24336
Lingley AJ, Mitchell DE, Crowder NA, Duffy KR (2019) Modification of Peak Plasticity Induced by Brief Dark Exposure. Neural Plast 2019: 1–10. https://doi.org/10.1155/2019/3198285
Erişir A, Van Horn SC, Sherman SM (1998) Distribution of synapses in the lateral geniculate nucleus of the cat: Differences between laminae A and A1 and between relay cells and interneurons. J Comp Neurol 390: 247–255. https://doi.org/10.1002/(SICI)1096-9861(19980112)390:2<247::AID-CNE7>3.0.CO;2-1
LeVay S, Ferster D (1977) Relay cell classes in the lateral geniculate nucleus of the cat and the effects of visual deprivation. J Comp Neurol 172: 563–584. https://doi.org/10.1002/cne.901720402
Lehmkuhle S, Kratz KE, Mangel SC, Sherman SM (1980) Spatial and temporal sensitivity of X- and Y-cells in dorsal lateral geniculate nucleus of the cat. J Neurophysiol 43: 520–541. https://doi.org/10.1152/jn.1980.43.2.520
Sanderson K (1971) The projection of the visual field to the lateral geniculate and medial interlaminar nuclei in the cat. J Comp Neurol 143: 101-108. https://doi.org/10.1002/cne.901430107
Tusa RJ, Rosenquist AC, Palmer LA (1979) Retinotopic organization of areas 18 and 19 in the cat. J Comp Neurol 185: 657–678. https://doi.org/10.1002/cne.901850405
Tusa RJ, Palmer LA (1980) Retinotopic organization of areas 20 and 21 in the cat. J Comp Neurol 193: 147–164. https://doi.org/10.1002/cne.901930110
Cullheim S (1978) Relations between cell body size, axon diameter and axon conduction velocity of cat sciatic α-motoneurons stained with horseradish peroxidase. Neurosci Lett 8: 17–20. https://doi.org/10.1016/0304-3940(78)90090-3
Davis WJ (1971) Functional significance of motorneuron size and soma position in swimmeret system of the lobster. J Neurophysiol 34: 274–288. https://doi.org/10.1152/jn.1971.34.2.274
Kovac MP, Davis WJ, Matera E, Gillette R (1982) Functional and structural correlates of cell size in paracerebral neurons of Pleurobranchaea californica. J Neurophysiol 47: 909–927. https://doi.org/10.1152/jn.1982.47.5.909
Fitzgibbon T, Wingate RJ, Thompson ID (1996) Soma and axon diameter distributions and central projections of ferret retinal ganglion cells. Vis Neurosci 13: 773–786. https://doi.org/10.1017/S0952523800008646
ACKNOWLEDGMENTS
The authors are grateful to the associates of the Neuromorphology Laboratory for their help in conducting the experiments.
Funding
This work was supported by the State Program 47 SP “Scientific and Technological Development of the Russian Federation” (2019–2030), theme reg. no. 0134-2019-0006.
Author information
Authors and Affiliations
Contributions
Conceptualization and experimental design (A.А.M., N.S.M.); data collection and processing (A.A.M., N.I.N.); statistical data treatment (А.А.M.); data analysis and interpretation (A.А.M., N.S.M.); writing and editing the manuscript (A.А.M., N.S.M.).
Corresponding author
Ethics declarations
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest.
Additional information
Translated by A. Polyanovsky
Russian Text © The Author(s), 2022, published in Zhurnal Evolyutsionnoi Biokhimii i Fiziologii, 2022, Vol. 58, No. 6, pp. 580–588https://doi.org/10.31857/S0044452922060079.
Rights and permissions
About this article
Cite this article
Mikhalkin, A.A., Nikitina, N.I. & Merkulyeva, N.S. Age-Related Changes in Soma Size of Y Neurons in the Cat Dorsal Lateral Geniculate Nucleus: Dorsoventral and Centroperipheral Gradients. J Evol Biochem Phys 58, 1809–1818 (2022). https://doi.org/10.1134/S0022093022060126
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022093022060126