Abstract
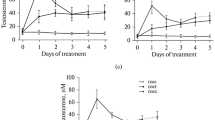
In type 1 diabetes mellitus (DM1), testicular synthesis of testosterone is impaired, leading to androgen insufficiency and disorders of spermatogenesis. Long-term use of high gonadotropin doses for the correction of these abnormalities leads to a decrease in the sensitivity of luteinizing hormone/human chorionic gonadotropin (LH/hCG) receptors in Leydig cells. The aim of this work was to study the effect of 3-day treatment of DM1 (streptozotocin-induced, 45 mg/kg) male Wistar rats with the allosteric LH/hCG receptor agonist 5-amino-N-tert-butyl-2-(methylsulfanyl)-4-(3-(nicotinamido)phenyl)thieno[2,3-d]pyrimidine-6-carboxamide (TP03, 15 mg/kg per day) on the effects of a relatively low hCG dose (10 IU/rat, single dose, s.c.) on blood testosterone levels, expression of steroidogenesis genes, and morphometric parameters of the seminiferous tubules. Pretreatment of rats with TP03 enhanced the stimulating effect of hCG on blood testosterone levels. This effect was produced, on the one hand, by enhanced steroidogenesis due to an increase in the expression of the Cyp11a1 gene, which encodes the mitochondrial cytochrome P450 side-chain cleavage enzyme (P450scc) responsible for the first stage of testosterone synthesis, and, on the other hand, by improved testicular sensitivity to gonadotropins due to an increase in the testicular LH/hCG receptor content in DM1 rats. In addition, pretreatment of DM1 rats with TP03 followed by hCG stimulation resulted in a more pronounced improvement in the morphometric parameters of the seminiferous tubules as compared to the groups that received TP03 or hCG alone. Thus, TP03 enables to increase the effectiveness of the hCG steroidogenic effect and reduce the dose of gonadotropin, which compensates for an androgen deficiency in diabetes.


Similar content being viewed by others
REFERENCES
Maresch CC, Stute DC, Alves MG, Oliveira PF, de Kretser DM, Linn T (2018) Diabetes-induced hyperglycemia impairs male reproductive function: a systematic review. Hum Reprod Update 24(1): 86–105. https://doi.org/10.1093/humupd/dmx033
Rato L, Alves MG, Duarte AI, Santos MS, Moreira PI, Cavaco JE, Oliveira PF (2015) Testosterone deficiency induced by progressive stages of diabetes mellitus impairs glucose metabolism and favors glycogenesis in mature rat Sertoli cells. Int J Biochem Cell Biol 66: 1–10. https://doi.org/10.1016/j.biocel.2015.07.001
Skurikhin EG, Pakhomova AV, Pershina OV, Krupin VA, Ermakova NN, Pan ES, Kudryashova AI, Ermolaeva LA, Khmelevskaya ES, Goldberg VE, Zhdanov VV, Dygai AM (2017) Role of Sertoli and Leydig cells in the regulation of spermatogonial stem cell and development of reproductive disorders in male C57Bl/6 mice with type 1 diabetes mellitus. Bull Exp Biol Med 64(2): 127–131. https://doi.org/10.1007/s10517-017-3940-6
Khamis T, Abdelalim AF, Abdallah SH, Saeed AA, Edress NM, Arisha AH (2020) Early intervention with breast milk mesenchymal stem cells attenuates the development of diabetic-induced testicular dysfunction via hypothalamic Kisspeptin/Kiss1r-GnRH/GnIH system in male rats. Biochim Biophys Acta Mol Basis Dis 866(1): 165577. https://doi.org/10.1016/j.bbadis.2019.165577
Wagner IV, Klöting N, Savchuk I, Eifler L, Kulle A, Kralisch-Jäcklein S, Dötsch J, Hiort O, Svechnikov K, Söder O (2021) Diabetes type 1 negatively influences Leydig cell function in rats, which is partially reversible by insulin treatment. Endocrinology 162(4): bqab017. https://doi.org/10.1210/endocr/bqab017
Barsiah S, Behnam-Rassouli M, Shahabipour F, Rostami S, Sabbaghi MA, Momeni Z, Tavassoli A, Sahebkar A (2019) Evaluation of testis hormonal and histopathological alterations in type I and type II diabetic rats. J Cell Biochem 120(10): 16775–16785. https://doi.org/10.1002/jcb.28936
Keyhanmanesh R, Hamidian G, Alipour MR, Oghbaei H (2019) Beneficial treatment effects of dietary nitrate supplementation on testicular injury in streptozotocin-induced diabetic male rats. Reprod Biomed Online 39(3): 357–371. https://doi.org/10.1016/j.rbmo.2018.11.027
Veldhuis JD, Liu PY, Takahashi PY, Keenan DM (2012) Dynamic testosterone responses to near-physiological LH pulses are determined by the time pattern of prior intravenous LH infusion. Am J Physiol Endocrinol Metab 303: 720–728. https://doi.org/10.1152/ajpendo.00200.2012
van Koppen CJ, Zaman GJ, Timmers CM, Kelder J, Mosselman S, van de Lagemaat R, Smit MJ, Hanssen RG (2008) A signaling-selective, nanomolar potent allosteric low molecular weight agonist for the human luteinizing hormone receptor. Naunyn Schmiedebergs Arch Pharmacol 378(5): 503–514. https://doi.org/10.1007/s00210-008-0318-3
Derkach KV, Dar’in DV, Bakhtyukov AA, Lobanov PS, Shpakov AO (2016) In vitro and in vivo studies of functional activity of new low molecular weight agonists of the luteinizing hormone receptor. Biochemistry (Mosc) Suppl Ser A: Membr Cell Biol 10(4): 294–300. https://doi.org/10.1134/S1990747816030132
Bakhtyukov AA, Derkach KV, Stepochkina AM, Shpakov AO, Dar’in DV (2019) A low molecular weight agonist of the luteinizing hormone receptor stimulates adenylyl cyclase in the testicular membranes and steroidogenesis in the testes of rats with type 1 diabetes. Biochemistry (Mosc) Suppl Ser A: Membr Cell Biol 13(4): 301–309. https://doi.org/10.1134/S1990747819040032
Bakhtyukov AA, Derkach KV, Gureev MA, Dar’in DV, Sorokoumov VN, Romanova IV, Morina IY, Stepochkina AM, Shpakov AO (2020) Comparative study of the steroidogenic effects of human chorionic gonadotropin and thieno[2,3-D]pyrimidine-based allosteric agonist of luteinizing hormone receptor in young adult, aging and diabetic male rats. Int J Mol Sci 21(20): 7493. https://doi.org/10.3390/ijms21207493
Bakhtyukov AA, Derkach KV, Sorokoumov VN, Stepochkina AM, Romanova IV, Morina IY, Zakharova IO, Bayunova LV, Shpakov AO (2022) The effects of separate and combined treatment of male rats with type 2 diabetes with metformin and orthosteric and allosteric agonists of luteinizing hormone receptor on steroidogenesis and spermatogenesis. Int J Mol Sci 23(1): 198. https://doi.org/10.3390/ijms23010198
Newton CL, Whay AM, McArdle CA, Zhang M, van Koppen CJ, van de Lagemaat R, Segaloff DL, Millar RP (2011) Rescue of expression and signaling of human luteinizing hormone G protein-coupled receptor mutants with an allosterically binding small-molecule agonist. Proc Natl Acad Sci USA 108(17): 7172–7176. https://doi.org/10.1073/pnas.1015723108
Mardanshahi T, Rezaei N, Zare Z, Malekzadeh Shafaroudi M, Mohammadi H (2018) Effects of L-Carnitine on the sperm parameters disorders, apoptosis of spermatogenic cells and testis histopathology in diabetic Rats. Int J Reprod Biomed 17(5): 325–336. https://doi.org/10.18502/ijrm.v17i5.4600
Pourheydar B, Azarm F, Farjah G, Karimipour M, Pourheydar M (2022) Effect of silymarin and metformin on the sperm parameters and histopathological changes of testes in diabetic rats: An experimental study. Int J Reprod Biomed 19(12): 1091–1104. https://doi.org/10.18502/ijrm.v19i12.10060
Omolaoye TS, Skosana BT, du Plessis SS (2018) Diabetes mellitus- induction: Effect of different streptozotocin doses on male reproductive parameters. Acta Histochem 120(2): 103–109. https://doi.org/10.1016/j.acthis.2017.12.005
He Z, Yin G, Li QQ, Zeng Q, Duan J (2021) Diabetes mellitus causes male reproductive dysfunction: a review of the evidence and mechanisms. In Vivo 35(5): 2503–2511. https://doi.org/10.21873/invivo.12531
Shoorei H, Khaki A, Shokoohi M, Khaki AA, Alihemmati A, Moghimian M, Abtahi-Eivary SH (2020) Evaluation of carvacrol on pituitary and sexual hormones and their receptors in the testicle of male diabetic rats. Hum Exp Toxicol 39(8): 1019–1030. https://doi.org/10.1177/0960327120909525
Dombroski BA, Nayak RR, Ewens KG, Ankener W, Cheung VG, Spielman RS (2010) Gene expression and genetic variation in response to endoplasmic reticulum stress in human cells. Am J Hum Genet 86(5): 719–729. https://doi.org/10.1016/j.ajhg.2010.03.017
Shiraishi K, Matsuyama H (2017) Gonadotoropin actions on spermatogenesis and hormonal therapies for spermatogenic disorders [Review]. Endocr J 64(2): 123–131. https://doi.org/10.1507/endocrj.EJ17-0001
Fink J, Schoenfeld BJ, Hackney AC, Maekawa T, Horie S (2021) Human chorionic gonadotropin treatment: a viable option for management of secondary hypogonadism and male infertility. Expert Rev Endocrinol Metab 16(1): 1–8. https://doi.org/10.1080/17446651.2021.1863783
Funding
This work was supported by the Russian Science Foundation (project no. 19-75-20122). Biochemical and molecular biological studies were carried out on the basis of the Center for Collective Use of Scientific Equipment for Physiological, Biochemical and Molecular Biological Research at the Sechenov Institute of Evolutionary Physiology and Biochemistry.
Author information
Authors and Affiliations
Contributions
Conceptualization and design of the experiment (A.O.S., K.V.D., V.N.S., A.A.B.), data collection (A.A.B., I.V.R., I.Y.M), data processing (A.A.B., I.V.R., K.V.D., I.Y.M.), manuscript writing and editing (A.A.B., I.V.R., K.V.D., A.O.S.).
Corresponding author
Ethics declarations
CONFLICT OF INTEREST
The authors declare that they no conflict of interest related to the publication of this article.
Additional information
Translated by A. Polyanovsky
Russian Text © The Author(s), 2022, published in Rossiiskii Fiziologicheskii Zhurnal imeni I.M. Sechenova, 2022, Vol. 108, No. 9, pp. 1175–1187https://doi.org/10.31857/S0869813922090023.
Rights and permissions
About this article
Cite this article
Bakhtyukov, A.A., Morina, I.Y., Derkach, K.V. et al. Development of Approaches to Reducing the Effective Gonadotropin Dose in Treating Androgen Insufficiency in Male Rats with Type 1 Diabetes Mellitus. J Evol Biochem Phys 58, 1503–1513 (2022). https://doi.org/10.1134/S0022093022050209
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022093022050209