Abstract
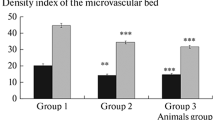
Changes in acetylcholine (ACh)-mediated dilation of the pial arteries in the sensorimotor cortex were studied in Sprague–Dawley rats after the formation of metabolic and hormonal disorders similar to those in type 2 diabetes mellitus (DM2). For this purpose, the rats were kept on a high-fat diet (HFD) for 2 months, and then some of them were injected with a low dose of streptozotocin (STZ, 35 mg/kg). Next, all animals were again receiving high-fat foods for another one month (a total of a 3-month HFD). The responses of pial arteries to the effects of acetylcholie (ACh, 10–7 M) alone or against the background of L-NAME, a non-selective nitric oxide synthase (NOS) blocker, or aminoguanidine (AG), a selective inducible nitric oxide synthase (iNOS) blocker, were assessed using intravital microscopy imaging technique. It was found that a 3-month HFD led to the development of endothelial dysfunction in the pial arteries of the sensorimotor cortex: the number of vessels dilated to the effect of ACh in the HFD group was 1.2–1.6 times smaller compared to the control group. ACh-mediated vascular dilation was endothelial NOS (eNOS)-depended only in the arteries with a caliber < 40 µm. In HFD rats, iNOS was not detected in the cerebral arteries. Animals with an STZ-induced DM2 model also developed endothelial dysfunction in the cerebral arteries: the number of vessels dilated to the effect of ACh in the DM2 group was 1.6–2.3 times smaller compared to the control group. In DM2 rats, the eNOS-associated signaling cascade did not control arterial reactivity, and vascular tone was mainly sustained due to iNOS-mediated reactions. In DM2 rats, the major abnormalities in the vascular dilatory response included the least number of vessels dilated in response to ACh alone with a significant decrease in the degree of dilation (by 1.5–1.6 times vs control), persistent ACh-mediated vascular dilation despite the presence of L-NAME, and the largest number of vascular constrictions in response to AG (60–70% of all the vessels examined). All these disorders were revealed in the pial arteries with a caliber < 40 µm, i.e. exactly in the vasculature segment that is maximally involved in blood–tissue gas exchange.




Similar content being viewed by others
REFERENCES
Gustafson B, Hammarstedt A, Andersson C, Smith U (2007) Inflamed adipose tissue: a culprit underlying the metabolic syndrome and atherosclerosis. Arterioscler Thromb Vasc Biol 27(11): 2276–2283. https://doi.org/10.1161/ATVBAHA.107.147835
Yang Q, Vijayakumar A, Kahn B (2018) Metabolites as regulators of insulin sensitivity and metabolism. Nat Rev Mol Cell Biol 19(10): 654–672. https://doi.org/10.1038/s41580-018-0044-8
Begum N (2003) Insulin signaling in the vasculature. Front Biosci 8: 796–804. https://doi.org/10.2741/1146
Weber S, Patel R, Lutsep H (2018) Cerebral amyloid angiopathy: diagnosis and potential therapies. Expert Rev Neurother 18(6): 503–513. https://doi.org/10.1080/14737175.2018.1480938
Madonna R, Balistreri C, Geng Y, Caterina R (2017) Diabetic microangiopathy: pathogenetic insights and novel therapeutic approaches. Vascular Pharmac 90: 1–7. https://doi.org/10.1016/j.vph.2017.01.004
Shi Y, Vanhoutte P (2017) Macro- and microvascular endothelial dysfunction in diabetes. J Diabetes 9: 434–449. https://doi.org/10.1111/1753-0407.12521
Elhessy H, Eltahry H, Erfan O, Mahdi M, Hazem N, El-Shahat M (2020) Evaluation of the modulation of nitric oxide synthase expression in cerebellum of diabetic albino rats and the possible protective effect of ferulic acid. Acta Histochem 122(8): 151633. https://doi.org/10.1016/j.acthis.2020.151633
Kaydash OA, Ivanov VV, Vengerovsky AI, Buyko EE, Schepetkin IA (2020) The experimental model of type 2 diabetes mellitus caused by a high-fat diet with low-dose streptozotocin in rats. Bull Siber Med 19(2): 41–47. https://doi.org/10.20538/1682-0363-2020-2-41-47
Srinivasan K, Viswanad B, Asrat L, Kaul C, Ramarao P (2005) Combination of high-fat diet-fed and low-dose streptozotocin-treated rat: A model for type 2 diabetes and pharmacological screening. Pharmacol Res 52: 313–320. https://doi.org/10.1016/j.phrs.2005.05.004
Yakimovich IY, Borodin DA, Podrezov IK, Ivanov VV, Vasilyev VN, Kotlovsky MY, Borisova LV, Milto IV (2015) White adipose tissue morphometric characteristics in hi-fat diet rats. Modern Probl Sci Educat 5: 1. (In Russ).
Shuvaeva VN, Gorshkova OP (2021) Age Changes in the Contribution of NO and Potassium Channels to the Dilation of the Pial Arterial Vessels in Rats. Russ J Physiol 107(11): 1440–1452. https://doi.org/10.31857/S0869813921110091
Domingueti C, Dusse L, Carvalho M, Sousa L, Gomes K, Fernandes A (2016) Diabetes mellitus: the linkage between oxidative stress, inflammation, hypercoagulability and vascular complications. J Diabetes Complicat 30: 738–745. https://doi.org/10.1016/j.jdiacomp.2015.12.018
Haratz S, Tanne D (2011) Diabetes, hyperglycemia and the management of cerebrovascular disease. Current Opinion Neurol 24(1): 81–88. https://doi.org/10.1097/WCO.obo13e3283418fed
Ding Y, Vaziri N, Coulson R, Kamanna V, Roh D (2000) Effects of stimulated hyperglycemia, insulin, and glucagon on endothelial nitric oxide synthase expression. Am J Physiol Endocrinol Metab 279(1): E11–E17. https://doi.org/10.1152/ajpendo.2000.279.1.E11
Arshad N, Lin T, Yahaya M (2018) Metabolic syndrome and its effect on the brain: possible mechanism. CNS Neurol Disord Drug Targeta 17(8): 595–603. https://doi.org/10.2174/1871527317666180724143258
Zhou H, Zhang X, Lu J (2014) Progress on diabetic cerebrovascular diseases. Bosn J Basic Med Sci 14(4): 185–190. https://doi.org/10.17305/bjbms.2014.4.203
Hein T, Omae T, Xu W, Yoshida A, Kuo L (2020) Role of arginase in selective impairment of endothelium-dependent nitric oxide synthase-mediated dilation of retinal arterioles during early diabetes. Invest Ophthalmol Visual Sci 61(5): 36. https://doi.org/10.1167/iovs.61.5.36
Kadoi Y, Goto F (2007) Effect of selective iNOS inhibition on systemic hemodynamics and mortality rate on endotoxic shock in streptozotocin-induced diabetic rats. Shock 28(5): 602-609. https://doi.org/10.1097/SHK.0b013e31804d452d
Toda N, Imamura T, Okamura T (2010) Alteration of nitric oxide-mediated blood flow regulation in diabetes mellitus. Pharmacol Ther 127(3): 189–209. https://doi.org/10.1016/j.pharmthera.2010.04.009
Vicent D, Ilany J, Kondo T, Naruse K, Fisher S, Kisanuki Y, Bursell S, Yanagisawa M, King G, Kahn C (2003) The role of endothelial insulin signaling in the regulation of vascular tone and insulin resistance. J Clin Invest 111: 1373–1380. https://doi.org/10.1172/JCI15211
Fateeva VV, Vorob’eva OV (2017) Nitric oxide: from the mechanism of action to pharmacological effects in cerebrovascular diseases. Zhurn Nevrol Psikhiatr im SS Korsakova 117(10): 131–135. https://doi.org/10.17116/jnevro2017117101131-135
Strunk V, Hahnenkamp K, Schneuing M, Schneuing M, Fischer L, Rich G (2001) Selective iNOS inhibition prevents hypotension in septic rats while preserving endothelium-dependent vasodilation. Anesth Anal 92: 681–687. https://doi.org/10.1213/00000539-200103000-00025
Antosova M, Strapkova A, Mikolka P, Mokry J, Medvedova I, Mokra D (2015) The influence of L-NAME on iNOSexpression and markers of oxidative stressin allergen-induced airway hyperreactivitym. Advs Exp Med Biol—Neurosci Respirat 7: 1–10. https://doi.org/10.1007/5584_2014_62
Nassi A, Malorgio F, Tedesco S, Cignarella A, Gaion R (2016) Upregulation of inducible NO synthase by exogenous adenosine in vascular smooth muscle cells activated by inflammatory stimuli in experimental diabetes. Cardiovasc Diabetol 15: 32. https://doi.org/10.1186/s12933-016-0349-x
Barrett E, Liu Z, Khamaisi M, King G, Klein R, Klein B, Hughes T, Craft S, Freedman B, Bowden D, Vinik A, Casellini C (2017) Diabetic microvascular disease: an endocrine society scientific statement. J Clin Endocrinol Metab 102(12): 4343–4410.
Funding
This study was state budget funded and supported by the State Program 47 GP “Scientific and Technological Development of the Russian Federation” (2019–2030), theme no. 0134-2019-0001.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
CONFLICT OF INTEREST
The author declares that she has no conflict of interest that might be associated with the publication of this article.
Additional information
Translated by A. Polyanovsky
Russian Text © The Author(s), 2022, published in Rossiiskii Fiziologicheskii Zhurnal imeni I.M. Sechenova, 2022, Vol. 108, No. 9, pp. 1148–1158https://doi.org/10.31857/S0869813922090096.
Rights and permissions
About this article
Cite this article
Sokolova, I.B. Involvement of Inducible Nitric Oxide Synthase in Pial Arterial Tone Formation under Metabolic Disorders and Streptozotocin-Induced Diabetes in Rats Kept on a High-Fat Diet. J Evol Biochem Phys 58, 1482–1490 (2022). https://doi.org/10.1134/S0022093022050180
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022093022050180