Abstract
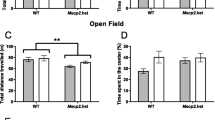
There are now a growing number of observations on the transgenerational impact of paternal stress on various offspring functions without involving direct interaction between offspring and father. In the offspring of parents who suffered from posttraumatic stress disorder (PTSD) have been observed an increase in PTSD-like symptoms. In experiments where a chronically elevated level of corticosterone was created in male mice during the entire period of spermatogenesis, a decrease in the stress reactivity of the hypothalamic–pituitary–adrenal (HPA) axis in the offspring of both sexes was shown. However, PTSD in patients creates a reduced level of glucocorticoids in the blood, and there are no experimental data on the effect of modeling PTSD in fathers on the activity of HPA axis of the offspring. The current study examined the relative influences of PTSD-like status (“stress–restress” paradigm) and depression-like status (“learned helplessness” paradigm) in male rats before mating on the HPA axis activity of their adult offspring. In addition to HPA axis activity, the expression of glucocorticoid receptors (GR) in the offspring was analyzed by quantitative immunocytochemistry in the hippocampus and medial prefrontal cortex (mPFC). We demonstrated that in female offspring of fathers with a PTSD-like or depressive-like status there is a decrease in basal and stress activity of the HPA axis in response to 30-min immobilization, which was accompanied by an increase in GR expression in the hippocampus and the 2nd layer mPFC. A similar profile of HPA axis activity was found in male offspring of fathers with modeling of PTSD. However, in male offspring of fathers with a depression-like status, the sensitivity of HPA axis to feedback signals decreased and was accompanied by a decrease in GR expression in the hippocampus and the 2nd layer of mPFC. We concluded that the PTSD- or depression-like status of male rats during the period of spermatogenesis has a differential effect on HPA axis activity and GR expression in the brain of their male offspring.


Similar content being viewed by others
REFERENCES
Yehuda R, Teicher MH, Seckl JR, Grossman RA, Morris A, Bierer LM (2007) Parental posttraumatic stress disorder as a vulnerability factor for low cortisol trait in offspring of Holocaust survivors. Archiv Gen Psychiatry 64: 1040–1048. https://doi.org/10.1001/archpsyc.64.9.1040
Yehuda R, Blair W, Labinsky E, Bierer LM (2007) Effects of parental PTSD on the cortisol response to dexamethasone administration in their adult offspring. Am J Psychiatry164: 163–166. https://doi.org/10.1176/ajp.2007.164.1.163
Rodgers AB, Morgan CP, Bronson SL, Revello S, Bale TL (2013) Paternal stress exposure alters sperm microRNA content and reprograms offspring HPA stress axis regulation. J Neurosci 33: 9003–9012. https://doi.org/10.1523/JNEUROSCI.0914-13.2013
Rodgers AB, Bale TL (2015) Germ cell origins of Posttraumatic stress disorder risk: the transgenerational impact of parental stress experience. Biol Psychiatry 78: 307–314. https://doi.org/10.1016/j.biopsych.2015.03.018
Ordyan NE, Pivina SG, Akulova VK, Kholova GI (2021) Changes in the nature of behavior and the activity of the hypophyseal-adrenocortical system in the offspring of paternal rats subjected to stress in the stress–restress paradigm before mating. Neurosci Behav Physiol 51: 528–534. https://doi.org/10.1007/s11055-021-01100-7
Andreasen NC (2011) What is post-traumatic stress disorder? Dialog Clin Neurosci 13: 240–243. https://doi.org/10.31887/DCNS.2011.13.2/nandreasen
Whirledge S, Cidlowski JA (2017) Glucocorticoids and reproduction: traffic control on the road to reproduction. Trends Endocrinol Metab 28: 399–415. https://doi.org/10.1016/j.tem.2017.02.005
Pivina SG, Rakitskaya VV, Akulova VK, Ordyan NE (2016) Activity of the hypothalamic–pituitary–adrenal system in prenatally stressed male rats on the experimental model of post-traumatic stress disorder. Bull Exp Biol Med 160: 601–604. https://doi.org/10.1007/s10517-016-3227-3
Czén B, Fuchs E, Wiborg O, Simon M (2016) Animal models of major depression and their clinical implications. Progress Neuropsyhopharmacol Biol Psychiatry 64: 293–310. https://doi.org/10.1016/j.pnpbp.2015.04.004
Mironova V, Pivina SG, Rybnikova E (2013) Effect of inescapable stress in rodent models of depression and posttraumatic stress disorder on CRH and vasopressin immunoreactivity in the hypothalamic paraventricular nucleus. Acta Physiol Hungar 100: 395–410. https://doi.org/10.1556/APhysiol.100.2013.4.4
McEwen BS, Nasca C, Gray JD (2016) Stress effects on neuronal structure: hippocampus, amygdala, and prefrontal cortex. Neuropsychopharmacology 41: 3–23. https://doi.org/10.1038/npp.2015.171
Ordyan NE, Pivina SG, Baranova KA, Rakitskaya VV, Akulova VK, Kholova GI (2021) Sex-dependent actions of prenatal stress on the activity of the hypothalamo-hypophyseal-adrenocortical system in rats: the role of corticosteroid receptors in the brain. Neurosci Behav Physiol 51: 357–366. https://doi.org/10.1007/s11055-021-01079-1
Boos A, Kohtes J, Janssen V, Mülling C, Stelljes A, Zerbe H, Hässig M, Thole HH (2006) Pregnancy effects on distribution of progesterone receptors, oestrogen receptor alpha, glucocorticoid receptors, Ki-67 antigen and apoptosis in the bovine interplacentomal uterine wall and foetal membranes. Anim Reprod Sci 91: 55–76. https://doi.org/10.1016/j.anireprosci.2005.03.012
Solomon Z, Kotler M, Mikulincer M (1988) Combat-related posttrau-matic stress disorder among second generation Holocaust survivors: preliminary findings. Am J Psychiatry 145: 865–886. https://doi.org/10.1176/ajp.145.7.865
Yehuda R, Schmeidler J, Giller EL, Siever LJ, Binder-Brynes K (1998) Relationship between Posttraumatic stress disorder characteristics of Holocaust survivors and their adult offspring. Am J Psychiatry 155: 841–843. https://doi.org/10.1176/ajp.155.6.841
Yehuda R, Schmeidler J, Wainberg M, Binder-Brynes K, Duvdevani T (1998) Vulnerability to posttraumatic stress disorder in adult offspring of Holocaust survivors. Am J Psychiatry 155: 1163–1171. https://doi.org/10.1176/ajp.155.9.1163
Yehuda R, Bell A, Bierer LM, Schmeidler J (2008) Maternal, not paternal, PTSD is related to increased risk for PTSD in offspring of Holocaust survivors. J Psychiatr Res 42: 1104–1111. https://doi.org/10.1016/j.jpsychires.2008.01.002
Cohen H, Zohar J, Gidron Y, Matar MA, Belkind D, Loewenthal U, Kozlovsky N, Kaplan Z (2006) Blunted HPA axis response to stress influences susceptibility to posttraumatic stress response in rats. Biol Psychiatry 59: 1208–1218. https://doi.org/10.1016/j.biopsych.2005.12.003
Danan D, Todder D, Zohar J, Cohen H (2021) Is PTSD-phenotype associated with HPA-axis sensitivity? Feedback inhibition and other modulating factors of glucocorticoid signaling dynamics. Int J Mol Sci 22: 6050. https://doi.org/10.3390/ijms22116050
Radley JJ, Gosselink KL, Sawchenko PE (2009) A discrete GABAergic relay mediates medial prefrontal cortical inhibition of the neuroendocrine stress response. J Neurosci 29: 7330–7340. https://doi.org/10.1523/JNEUROSCI.5924-08.2009
Gabbott PL, Warner TA, Jays PR, Salway P, Busby SJ (2005) Prefrontal cortex in the rat: projections to subcortical autonomic, motor, and limbic centers. J Comp Neurol 492: 145–177. https://doi.org/10.1002/cne.20738
Radley JJ, Rocher AB, Rodriguez A, Ehlenberger DB, Dammann M, McEwen BS, Morrison JH, Wearne SL, Hof PR (2008) Repeated stress alters dendritic spine morphology in the rat medial prefrontal cortex. J Compar Neurol 507(1): 1141–1150. https://doi.org/10.1002/cne.21588
Yehuda R, Daskalakis NP, Lehrner A, Desarnaud F, Bader HN, Makotkine I, Flory JD, Bierer LM, Meaney MJ (2014) Influences of maternal and paternal PTSD on epigenetic regulation of the glucocorticoid receptor gene in Holocaust survivor offspring. Am J Psychiatry 171: 872–880. https://doi.org/10.1176/appi.ajp.2014.13121571
Funding
This work was carried out as part of the Program “Basic Scientific Research for the Long-Term Development and Competitiveness of Society and State” (47_110_LTDaC).
Author information
Authors and Affiliations
Contributions
Idea of work and planning of the experiment—N.E.O., writing and editing the article—N.E.O., S.G.P., experimental animal preparation—G.I.K., V.K.A., data collection—V.V.R., S.G.P., G.I.K., data processing—V.K.A., G.I.K.
Corresponding author
Ethics declarations
CONFLICT OF INTEREST
The authors declare no apparent or potential conflicts of interest related to the publication of this article.
Additional information
Translated by A. Dyomina
Russian Text © The Author(s), 2022, published in Rossiiskii Fiziologicheskii Zhurnal imeni I.M. Sechenova, 2022, Vol. 108, No. 9, pp. 1114–1124https://doi.org/10.31857/S0869813922090114.
Rights and permissions
About this article
Cite this article
Ordyan, N.E., Pivina, S.G., Kholova, G.I. et al. Differential Effect of Male Rat’s PTSD-Like or Depression-Like Status before Mating on the Activity of the Hypothalamic–Pituitary–Adrenal Axis of Adult Offspring. J Evol Biochem Phys 58, 1455–1463 (2022). https://doi.org/10.1134/S0022093022050155
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022093022050155