Abstract
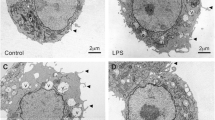
Inflammation and oxidative stress are well known to induce the biogenesis of lipid droplets (LDs) in various cell types, in which fatty acids are deposited as components of triglycerides (TGs) and/or cholesterol esters. This metabolic shift is critical for cell protection against the toxicity of excess lipids or their oxidative derivatives produced under various stressful exposures, including oxidative stress. The effect of bacterial lipopolysaccharide (LPS) on LD formation in CNS structures was mainly studied on microglial cells, whereas its effect on lipid metabolism in neurons, potentially associated with LD formation, has been studied neither on cell lines nor on primary neuronal cultures. This work was carried out on the PC12 neuronal cell line, which is widely used to explore the mechanisms of neuroinflammation and neurodegeneration, aiming to study the effects of LPS on LD formation, neutral lipid accumulation, and metabolism of major lipid classes, as well as to elucidate the mechanism mediating these effects. PC12 cell incubation with LPS for 24 h led to a significant accumulation of LDs and an increase in the TG level. Etomoxir, a carnitine palmitoyltransferase 1 (CPT1) inhibitor, also induced an increase in an absolute TG content. To elucidate the metabolic pathways of TG accumulation, PC12 cells were preincubated with [3H]-oleic acid and then examined for radioactive label incorporation into major lipid classes. LPS caused an increase the level of TG and free oleic acid radioactivity, accompanied by a significant inhibition of oleic acid oxidation and a decrease the radioactivity of phospholipids. PC12 cell incubation with LPS also caused a decrease in CPT1 expression. Our data indicate that in PC12 cells, LPS suppresses CPT1 expression and fatty acid oxidation, leading to sequestration of excess fatty acids into TG and the formation of LDs. Under conditions of the bacterial pathogen action, this mechanism seems to represent a cell survival strategy that protects from lipotoxicity.




Similar content being viewed by others
REFERENCES
Lukiw WJ (2020) Gastrointestinal (GI) Tract Microbiome-Derived Neurotoxins-Potent Neuro-Inflammatory Signals From the GI Tract via the Systemic Circulation Into the Brain. Front Cell Infect Microbiol 10: 22. https://doi.org/10.3389/fcimb.2020.00022
Zhao Y, Jaber V, Lukiw WJ (2017) Secretory Products of the Human GI Tract Microbiome and Their Potential Impact on Alzheimer’s Disease (AD): Detection of Lipopolysaccharide (LPS) in AD Hippocampus. Front Cell Infect Microbiol 7: 318. https://doi.org/10.3389/fcimb.2017.00318
Nikolaeva S, Bayunova L, Sokolova T, Vlasova Y, Bachteeva V, Avrova N, Parnova R (2015) GM1 and GD1a gangliosides modulate toxic and inflammatory effects of E. coli lipopolysaccharide by preventing TLR4 translocation into lipid rafts. Biochim Biophys Acta 1851 (3): 239–247. https://doi.org/10.1016/j.bbalip.2014.12.004
Wang H, Xu YS, Wang ML, Cheng C, Bian R, Yuan H, Wang Y, Guo T, Zhu LL, Zhou H (2017) Protective effect of naringin against the LPS-induced apoptosis of PC12 cells: Implications for the treatment of neurodegenerative disorders. Int J Mol Med 39 (4): 819–830. https://doi.org/10.3892/ijmm.2017.2904
Liu X, Xiao Q, Zhao K, Gao Y (2013) Ghrelin inhibits high glucose-induced PC12 cell apoptosis by regulating TLR4/NF-kappaB pathway. Inflammation 36 (6): 1286–1294. https://doi.org/10.1007/s10753-013-9667-2
Yang Q, Kang ZH, Zhang J, Qu F, Song B (2021) Neuroprotective Effects of Isoquercetin: An In Vitro and In Vivo Study. Cell J 23 (3): 355–365. https://doi.org/10.22074/cellj.2021.7116
Wu Z, Lu Z, Ou J, Su X, Liu J (2020) Inflammatory response and oxidative stress attenuated by sulfiredoxin1 in neuronlike cells depends on nuclear factor erythroid2related factor 2. Mol Med Rep 22 (6): 4734–4742. https://doi.org/10.3892/mmr.2020.11545
Bayunova LV, Parnova RG, Avrova NF (2015) Antiapoptotic effect of gangliosides on PC12 cells exposed to bacterial lipopolysaccharide. Zh Evol Biokhim Fiziol 51 (2): 88–94.
Wakulik K, Wiatrak B, Szczukowski L, Bodetko D, Szandruk-Bender M, Dobosz A, Swiatek P, Gasiorowski K (2020) Effect of Novel Pyrrolo[3,4-d]pyridazinone Derivatives on Lipopolysaccharide-Induced Neuroinflammation. Int J Mol Sci 21 (7): 2575. https://doi.org/10.3390/ijms21072575
Wiatrak B, Balon K (2021) Protective Activity of Abeta on Cell Cultures (PC12 and THP-1 after Differentiation) Preincubated with Lipopolysaccharide (LPS). Mol Neurobiol 58 (4): 1453–1464. https://doi.org/10.1007/s12035-020-02204-w
Feingold KR, Shigenaga JK, Kazemi MR, McDonald CM, Patzek SM, Cross AS, Moser A, Grunfeld C (2012) Mechanisms of triglyceride accumulation in activated macrophages. J Leukoc Biol 92 (4): 829–839. https://doi.org/10.1189/jlb.1111537
Fock E, Bachteeva V, Lavrova E, Parnova R (2018) Mitochondrial-Targeted Antioxidant MitoQ Prevents E. coli Lipopolysaccharide-Induced Accumulation of Triacylglycerol and Lipid Droplets Biogenesis in Epithelial Cells. J Lipids 2018: 5745790. https://doi.org/10.1155/2018/5745790
Wang J, Si Y, Wu C, Sun L, Ma Y, Ge A, Li B (2012) Lipopolysaccharide promotes lipid accumulation in human adventitial fibroblasts via TLR4-NF-kappaB pathway. Lipids Health Dis 11: 139. https://doi.org/10.1186/1476-511X-11-139
Bailey AP, Koster G, Guillermier C, Hirst EM, MacRae JI, Lechene CP, Postle AD, Gould AP (2015) Antioxidant Role for Lipid Droplets in a Stem Cell Niche of Drosophila. Cell 163 (2): 340–353. https://doi.org/10.1016/j.cell.2015.09.020
Lee SJ, Zhang J, Choi AM, Kim HP (2013) Mitochondrial dysfunction induces formation of lipid droplets as a generalized response to stress. Oxid Med Cell Longev 2013: 327167. https://doi.org/10.1155/2013/327167
Schonfeld P,Reiser G (2021) How the brain fights fatty acids’ toxicity. Neurochem Int 148: 105050. https://doi.org/10.1016/j.neuint.2021.105050
Krahmer N, Farese RV Jr, Walther TC (2013) Balancing the fat: lipid droplets and human disease. EMBO Mol Med 5 (7): 973–983. https://doi.org/10.1002/emmm.201100671
Shimabukuro MK, Langhi LG, Cordeiro I, Brito JM, Batista CM, Mattson MP, Mello Coelho V (2016) Lipid-laden cells differentially distributed in the aging brain are functionally active and correspond to distinct phenotypes. Sci Rep 6: 23795. https://doi.org/10.1038/srep23795
Yang DS, Stavrides P, Saito M, Kumar A, Rodriguez-Navarro JA, Pawlik M, Huo C, Walkley SU, Saito M, Cuervo AM, Nixon RA (2014) Defective macroautophagic turnover of brain lipids in the TgCRND8 Alzheimer mouse model: prevention by correcting lysosomal proteolytic deficits. Brain 137 (Pt 12): 3300–3318. https://doi.org/10.1093/brain/awu278
Girard V, Jollivet F, Knittelfelder O, Celle M, Arsac JN, Chatelain G, Van den Brink DM, Baron T, Shevchenko A, Kuhnlein RP, Davoust N, Mollereau B (2021) Abnormal accumulation of lipid droplets in neurons induces the conversion of alpha-Synuclein to proteolytic resistant forms in a Drosophila model of Parkinson’s disease. PLoS Genet 17 (11): e1009921. https://doi.org/10.1371/journal.pgen.1009921
Farmer BC, Walsh AE, Kluemper JC, Johnson LA (2020) Lipid Droplets in Neurodegenerative Disorders. Front Neurosci 14: 742. https://doi.org/10.3389/fnins.2020.00742
Ioannou MS, Jackson J, Sheu SH, Chang CL, Weigel AV, Liu H, Pasolli HA, Xu CS, Pang S, Matthies D, Hess HF, Lippincott-Schwartz J, Liu Z (2019) Neuron-Astrocyte Metabolic Coupling Protects against Activity-Induced Fatty Acid Toxicity. Cell 177 (6): 1522–1535 e14. https://doi.org/10.1016/j.cell.2019.04.001
Lee LL, Aung HH, Wilson DW, Anderson SE, Rutledge JC, Rutkowsky JM (2017) Triglyceride-rich lipoprotein lipolysis products increase blood-brain barrier transfer coefficient and induce astrocyte lipid droplets and cell stress. Am J Physiol Cell Physiol 312 (4): C500–C516. https://doi.org/10.1152/ajpcell.00120.2016
Liu L, MacKenzie KR, Putluri N, Maletic-Savatic M, Bellen HJ (2017) The Glia-Neuron Lactate Shuttle and Elevated ROS Promote Lipid Synthesis in Neurons and Lipid Droplet Accumulation in Glia via APOE/D. Cell Metab 26 (5): 719–737 e6. https://doi.org/10.1016/j.cmet.2017.08.024
Marschallinger J, Iram T, Zardeneta M, Lee SE, Lehallier B, Haney MS, Pluvinage JV, Mathur V, Hahn O, Morgens DW, Kim J, Tevini J, Felder TK, Wolinski H, Bertozzi CR, Bassik MC, Aigner L, Wyss-Coray T (2020) Lipid-droplet-accumulating microglia represent a dysfunctional and proinflammatory state in the aging brain. Nat Neurosci 23 (2): 194–208. https://doi.org/10.1038/s41593-019-0566-1
Khatchadourian A, Bourque SD, Richard VR, Titorenko VI, Maysinger D (2012) Dynamics and regulation of lipid droplet formation in lipopolysaccharide (LPS)-stimulated microglia. Biochim Biophys Acta 1821 (4): 607–617. https://doi.org/10.1016/j.bbalip.2012.01.007
Fock E, Lavrova E, Bachteeva V, Nikolaeva S, Parnova R (2019) Suppression of fatty acid beta-oxidation and energy deficiency as a cause of inhibitory effect of E. coli lipopolysaccharide on osmotic water transport in the frog urinary bladder. Comp Biochem Physiol C Toxicol Pharmacol 218: 81–87. https://doi.org/10.1016/j.cbpc.2019.01.001
Huang YL, Morales-Rosado J, Ray J, Myers TG, Kho T, Lu M, Munford RS (2014) Toll-like receptor agonists promote prolonged triglyceride storage in macrophages. J Biol Chem 289 (5): 3001–3012. https://doi.org/10.1074/jbc.M113.524587
Feingold KR, Wang Y, Moser A, Shigenaga JK, Grunfeld C (2008) LPS decreases fatty acid oxidation and nuclear hormone receptors in the kidney. J Lipid Res 49 (10): 2179–2187. https://doi.org/10.1194/jlr.M800233-JLR200
Przybytkowski E, Behrendt M, Dubois D, Maysinger D (2009) Nanoparticles can induce changes in the intracellular metabolism of lipids without compromising cellular viability. FEBS J 276 (21): 6204–6217. https://doi.org/10.1111/j.1742-4658.2009.07324.x
Wang F, Wang L, Sui G, Yang C, Guo M, Xiong X, Chen Z, Lei P (2021) IGF-1 Alleviates Mitochondrial Apoptosis through the GSK3beta/NF-kappaB/NLRP3 Signaling Pathway in LPS-Treated PC-12 Cells. J Mol Neurosci 71 (6): 1320–1328. https://doi.org/10.1007/s12031-020-01759-6
Xie Y, Zhang H, Zhang Y, Wang C, Duan D, Wang Z (2018) Chinese Angelica Polysaccharide (CAP) Alleviates LPS-Induced Inflammation and Apoptosis by Down-Regulating COX-1 in PC12 Cells. Cell Physiol Biochem 49 (4): 1380–1388. https://doi.org/10.1159/000493415
Ma S, Zhang C, Zhang Z, Dai Y, Gu R, Jiang R (2019) Geniposide protects PC12 cells from lipopolysaccharide-evoked inflammatory injury via up-regulation of miR-145-5p. Artif Cells Nanomed Biotechnol 47 (1): 2875–2881. https://doi.org/10.1080/21691401.2019.1626406
Huang H, Hong Q, Tan HL, Xiao CR, Gao Y (2016) Ferulic acid prevents LPS-induced up-regulation of PDE4B and stimulates the cAMP/CREB signaling pathway in PC12 cells. Acta Pharmacol Sin 37 (12): 1543–1554. https://doi.org/10.1038/aps.2016.88
Nikolaeva S, Sokolova T, Bayunova L, Parnova R (2013) Protective effect of GD1a and GM1 gangliosides against the toxic action of bacterial lipopolysaccharide on neuronal and epithelial cells. FEBS J 280 (Suppl 1): 433–433.
Kapetanovic R, Afroz SF, Ramnath D, Lawrence GM, Okada T, Curson JE, de Bruin J, Fairlie DP, Schroder K, St John JC, Blumenthal A, Sweet MJ (2020) Lipopolysaccharide promotes Drp1-dependent mitochondrial fission and associated inflammatory responses in macrophages. Immunol Cell Biol 98 (7): 528–539. https://doi.org/10.1111/imcb.12363
Harland M, Torres S, Liu J, Wang X (2020) Neuronal Mitochondria Modulation of LPS-Induced Neuroinflammation. J Neurosci 40 (8): 1756–1765. https://doi.org/10.1523/JNEUROSCI.2324-19.2020
Serviddio G, Giudetti AM, Bellanti F, Priore P, Rollo T, Tamborra R, Siculella L, Vendemiale G, Altomare E, Gnoni GV (2011) Oxidation of hepatic carnitine palmitoyl transferase-I (CPT-I) impairs fatty acid beta-oxidation in rats fed a methionine-choline deficient diet. PLoS One 6 (9): e24084. https://doi.org/10.1371/journal.pone.0024084
Filomeni G, De Zio D, Cecconi F (2015) Oxidative stress and autophagy: the clash between damage and metabolic needs. Cell Death Differ 22 (3): 377–388. https://doi.org/10.1038/cdd.2014.150
Schonfeld P, Reiser G (2013) Why does brain metabolism not favor burning of fatty acids to provide energy? Reflections on disadvantages of the use of free fatty acids as fuel for brain. J Cereb Blood Flow Metab 33 (10): 1493–1499. https://doi.org/10.1038/jcbfm.2013.128
Jernberg JN, Bowman CE, Wolfgang MJ, Scafidi S (2017) Developmental regulation and localization of carnitine palmitoyltransferases (CPTs) in rat brain. J Neurochem 142 (3): 407–419. https://doi.org/10.1111/jnc.14072
Edmond J, Robbins RA, Bergstrom JD, Cole RA, de Vellis J (1987) Capacity for substrate utilization in oxidative metabolism by neurons, astrocytes, and oligodendrocytes from developing brain in primary culture. J Neurosci Res 18 (4): 551–561. https://doi.org/10.1002/jnr.490180407
Stoll EA, Makin R, Sweet IR, Trevelyan AJ, Miwa S, Horner PJ, Turnbull DM (2015) Neural Stem Cells in the Adult Subventricular Zone Oxidize Fatty Acids to Produce Energy and Support Neurogenic Activity. Stem Cells 33 (7): 2306–2319. https://doi.org/10.1002/stem.2042
Bianchetti G, Di Giacinto F, De Spirito M, Maulucci G (2020) Machine-learning assisted confocal imaging of intracellular sites of triglycerides and cholesteryl esters formation and storage. Anal Chim Acta 1121: 57–66. https://doi.org/10.1016/j.aca.2020.04.076
Wolfgang MJ, Cha SH, Millington DS, Cline G, Shulman GI, Suwa A, Asaumi M, Kurama T, Shimokawa T, Lane MD (2008) Brain-specific carnitine palmitoyl-transferase-1c: role in CNS fatty acid metabolism, food intake, and body weight. J Neurochem 105 (4): 1550–1559. https://doi.org/10.1111/j.1471-4159.2008.05255.x
Ding Y, Zhang H, Liu Z, Li Q, Guo Y, Chen Y, Chang Y, Cui H (2021) Carnitine palmitoyltransferase 1 (CPT1) alleviates oxidative stress and apoptosis of hippocampal neuron in response to beta-Amyloid peptide fragment Abeta25-35. Bioengineered 12(1): 5440-5449. https://doi.org/10.1080/21655979.2021.1967032
Sierra AY, Gratacos E, Carrasco P, Clotet J, Urena J, Serra D, Asins G, Hegardt FG, Casals N (2008) CPT1c is localized in endoplasmic reticulum of neurons and has carnitine palmitoyltransferase activity. J Biol Chem 283 (11): 6878–6885. https://doi.org/10.1074/jbc.M707965200
Knobloch M, Pilz GA, Ghesquiere B, Kovacs WJ, Wegleiter T, Moore DL, Hruzova M, Zamboni N, Carmeliet P, Jessberger S (2017) A Fatty Acid Oxidation-Dependent Metabolic Shift Regulates Adult Neural Stem Cell Activity. Cell Rep 20 (9): 2144–2155. https://doi.org/10.1016/j.celrep.2017.08.029
ACKNOWLEDGMENTS
The authors are grateful to associates of the Institute’s Laboratory of Molecular Endocrinology and Neurochemistry for the provision of the PC12 cell line.
Funding
This study was implemented under the state assignment to the Sechenov Institute of Evolutionary Physiology and Biochemistry of the Russian Academy of Sciences (no. 075-00776-19-02).
Author information
Authors and Affiliations
Contributions
Conceptualization, experimental design (R.G.P.); data collection (S.D.N., E.M.F.); data processing (S.D.N., E.M.F., R.G.P.); writing and editing the manuscript (R.G.P.).
Corresponding author
Ethics declarations
CONFLICT OF INTEREST
The authors declare that they have neither evident nor potential conflict of interest related to the publication of this article.
Additional information
Translated by A. Polyanovsky
Russian Text © The Author(s), 2022, published in Rossiiskii Fiziologicheskii Zhurnal imeni I.M. Sechenova, 2022, Vol. 108, No. 7, pp. 903–916https://doi.org/10.31857/S086981392207007X.
Rights and permissions
About this article
Cite this article
Nikolaeva, S.D., Fock, E.M. & Parnova, R.G. Lipopolysaccharide Stimulates Triglyceride Accumulation and Lipid Droplet Biogenesis in PC12 Cells: the Role of Carnitine Palmitoyltransferase 1 Down-Regulation and Suppression of Fatty Acid Oxidation. J Evol Biochem Phys 58, 1152–1162 (2022). https://doi.org/10.1134/S0022093022040184
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022093022040184