Abstract
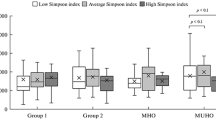
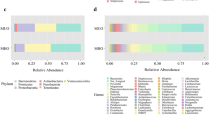
Obesity is associated with a gut microbiome imbalance and the risk of neurological complications. However, the risk of complications is determined by the metabolic type of obesity. The aim was to study the effect of intestinal microbiome taxa on the content of neurotrophins. 130 healthy non-obese donors and 104 obese patients were examined. Depending on the metabolic type of obesity, patients were divided into subgroups with metabolically healthy obesity (MHO, n = 40) and metabolically unhealthy obesity (MUHO, n = 55). Serum concentrations of neurotrophins were measured, and the gut microbiome qualitative assessment was determined by sequencing of the variable region of the 16S rRNA gene. Taxa with a positive correlation with brain-derived neurotrophic factor (BDNF) concentration were mainly represented by butyrate and/or GABA-producing microorganisms capable of degrading mucin. In healthy donors, a positive relationship was found between BDNF and S24-7. BDNF association with Bacteroides spp., Rikenellaceae, Oscillospira spp., [Barnesiellaceae], B. ovatus and Anaerostipes spp. was noted in MHO, while in MUHO with Bifidobacterium spp. and Coprococcus spp. Taxa negatively correlated with BDNF have also been identified, predominantly Gram-positive in obese patients. Nerve growth factor (NGF) concentration has been associated with the gut microbiome in obesity but not in healthy donors. A positive relationship with NGF has been noted for Odoribacter spp. in MHO patients and Slackia spp. in MUHO patients. H. parainfluenzae, Erysipelotrichaceae, Megamonas spp. and Clostridiaceae had a negative association on NGF level in MHO. In MUHO, a similar effect was shown by ML615J-287 and Clostridiales. Thus, obesity, regardless of metabolic type, is associated with the appearance of the “gut microbiome—NGF” relationship, which, apparently, is a consequence of increased intestinal permeability. The list of taxa that correlated with neurotrophins was unique for each group of patients. The obtained results indicate a significant effect ofinter-taxa interaction on the neurotrophin level.



Similar content being viewed by others
REFERENCES
Lee CJ, Sears CL, Maruthur N (2020) Gut microbiome and its role in obesity and insulin resistance. Ann N Y Acad Sci 1461: 37–52. https://doi.org/10.1111/nyas.14107
Singer-Englar T, Barlow G, Mathur R (2019) Obesity, diabetes, and the gut microbiome: an updated review. Expert Rev Gastroenterol Hepatol 13: 3–15. https://doi.org/10.1080/17474124.2019.1543023
Kotrova AD, Shishkin AN, Voropaeva LS, Lavrenova NS, Blind LA, Lukashenko MV, Ermolenko EI (2021) Gender assessment of the intestinal microbiome in obese patients.
Clinical Gastroenterology 194: 91–99. https://doi.org/10.31146/1682- 8658-ecg-194-10-91-99
Gaponov AM, Volkova NI, Ganenko LA, Naboka YUL, Markelova MI, Sinyagina MN, Kharchenko AM, Khusnutdinova DR, Rumyantsev SA, Tutelyan AV, Makarov BB, Yudin SM, Shestopalov AV (2021) Features of the colon microbiome in obese patients with its various phenotypes (original article). Journal of Microbiology, Epidemiology and Immunobiology 98: 144–155. https://doi.org/10.36233/0372-9311-66
Rosenbaum M, Knight R, Leibel RL (2015) The gut microbiota in human energy homeostasis and obesity. Trends Endocrinol Metab 26: 493–501. https://doi.org/10.1016/j.tem.2015.07.002
Hu J, Lin S, Zheng B, Cheung PCK (2018) Short-chain fatty acids in control of energy metabolism. Crit Rev Food Sci Nutr 58: 1243–1249.
Blaak EE, Canfora EE, Theis S, Frost G, Groen AK, Mithieux G, Nauta A, Scott K, Stahl B, van Harsselaar J, van Tol R, Vaughan EE, Verbeke K (2020) Short chain fatty acids in human gut and metabolic health. Benef Microbes 11: 411–455. https://doi.org/10.3920/BM2020.0057
Heiss CN, Olofsson LE (2019) The role of the gut microbiota in development, function and disorders of the central nervous system and the enteric nervous system. J Neuroendocrinol 31: 1–11. https://doi.org/10.1111/jne.12684
Sharon G, Sampson TR, Geschwind DH, Mazmanian SK (2016) The Central Nervous System and the Gut Microbiome. Cell 167: 915–932. https://doi.org/10.1016/j.cell.2016.10.027
Grasset E, Puel A, Charpentier J, Collet X, Christensen JE, Tercé F, Burcelin R (2017) A Specific Gut Microbiota Dysbiosis of Type 2 Diabetic Mice Induces GLP-1 Resistance through an Enteric NO-Dependent and Gut-Brain Axis Mechanism. Cell Metab 25: 1075–1090.e5. https://doi.org/10.1016/j.cmet.2017.04.013
Guillemot-Legris O, Muccioli GG (2017) Obesity-Induced Neuroinflammation: Beyond the Hypothalamus. Trends Neurosci 40: 237–253. https://doi.org/10.1016/j.tins.2017.02.005
Iacobini C, Pugliese G, Blasetti Fantauzzi C, Federici M, Menini S (2019) Metabolically healthy versus metabolically unhealthy obesity. Metabolism 92: 51–60. https://doi.org/10.1016/j.metabol.2018.11.009
Bercik P, Denou E, Collins J, Jackson W, Lu J, Jury J, Deng Y, Blennerhassett P, Macri J, McCoy KD, Verdu EF, Collins SM (2011) The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology 141: 599–609, 609.e1–3. https://doi.org/10.1053/j.gastro.2011.04.052
Schéle E, Grahnemo L, Anesten F, Hallén A, Bäckhed F, Jansson J-O (2013) The gut microbiota reduces leptin sensitivity and the expression of the obesity-suppressing neuropeptides proglucagon (Gcg) and brain-derived neurotrophic factor (Bdnf) in the central nervous system. Endocrinology 154: 3643–3651. https://doi.org/10.1210/en.2012-2151
Sudo N, Chida Y, Aiba Y, Sonoda J, Oyama N, Yu XN, Kubo C, Koga Y (2004) Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J Physiol 558: 263–275. https://doi.org/10.1113/jphysiol.2004.063388
Desbonnet L, Clarke G, Traplin A, O’Sullivan O, Crispie F, Moloney RD, Cotter PD, Dinan TG, Cryan JF (2015) Gut microbiota depletion from early adolescence in mice: Implications for brain and behaviour. Brain Behav Immun 48: 165–173. https://doi.org/10.1016/j.bbi.2015.04.004
Fröhlich EE, Farzi A, Mayerhofer R, Reichmann F, Jačan A, Wagner B, Zinser E, Bordag N, Magnes C, Fröhlich E, Kashofer K, Gorkiewicz G, Holzer P (2016) Cognitive impairment by antibiotic-induced gut dysbiosis: Analysis of gut microbiota-brain communication. Brain Behav Immun 56: 140–155. https://doi.org/10.1016/j.bbi.2016.02.020
Cui M, Xiao H, Li Y, Dong J, Luo D, Li H, Feng G, Wang H, Fan S (2017) Total abdominal irradiation exposure impairs cognitive function involving miR-34a-5p/BDNF axis. Biochim Biophys Acta—Mol Basis Dis 1863: 2333–2341. https://doi.org/10.1016/j.bbadis.2017.06.021
Stilling RM, Ryan FJ, Hoban AE, Shanahan F, Clarke G, Claesson MJ, Dinan TG, Cryan JF (2015) Microbes & neurodevelopment—Absence of microbiota during early life increases activity-related transcriptional pathways in the amygdala. Brain Behav Immun 50: 209–220. https://doi.org/10.1016/j.bbi.2015.07.009
Savignac HM, Corona G, Mills H, Chen L, Spencer JPE, Tzortzis G, Burnet PWJ (2013) Prebiotic feeding elevates central brain derived neurotrophic factor, N-methyl-d-aspartate receptor subunits and d-serine. Neurochem Int 63: 756–764. https://doi.org/10.1016/j.neuint.2013.10.006
O’Sullivan E, Barrett E, Grenham S, Fitzgerald P, Stanton C, Ross RP, Quigley EMM, Cryan JF, Dinan TG (2011) BDNF expression in the hippocampus of maternally separated rats: Does Bifidobacterium breve 6330 alter BDNF levels? Benef Microbes 2: 199–207. https://doi.org/10.3920/BM2011.0015
Sun J, Wang F, Hu X, Yang C, Xu H, Yao Y, Liu J (2018) Clostridium butyricum Attenuates Chronic Unpredictable Mild Stress-Induced Depressive-Like Behavior in Mice via the Gut-Brain Axis. J Agric Food Chem 66: 8415–8421. https://doi.org/10.1021/acs.jafc.8b02462
Borrelli L, Aceto S, Agnisola C, De Paolo S, Dipineto L, Stilling RM, Dinan TG, Cryan JF, Menna LF, Fioretti A (2016) Probiotic modulation of the microbiota-gut-brain axis and behaviour in zebrafish. Sci Rep 6: 1–9. https://doi.org/10.1038/srep30046
Cuomo M, Borrelli L, Monica R Della, Coretti L, De Riso G, Di Durazzo LDL, Fioretti A, Lembo F, Dinan TG, Cryan JF, Cocozza S, Chiariotti L (2021) DNA methylation profiles of Tph1a and BDNF in gut and brain of L. Rhamnosus-treated Zebrafish. Biomolecules 11: 1–13. https://doi.org/10.3390/biom11020142
Hwang YH, Park S, Paik JW, Chae SW, Kim DH, Jeong DG, Ha E, Kim M, Hong G, Park SH, Jung SJ, Lee SM, Na KH, Kim J, Chung YC (2019) Efficacy and safety of lactobacillus plantarum C29-fermented soybean (DW2009) in individuals with mild cognitive impairment: A 12-week, multi-center, randomized, double-blind, placebo-controlled clinical trial. Nutrients 11(2): 305. https://doi.org/10.3390/nu11020305
Li C, Cai YY, Yan ZX (2018) Brain-derived neurotrophic factor preserves intestinal mucosal barrier function and alters gut microbiota in mice. Kaohsiung J Med Sci 34: 134–141. https://doi.org/10.1016/j.kjms.2017.11.002
Steinkamp M, Schulte N, Spaniol U, Pflüger C, Hartmann C, Kirsch J, von Boyen GB (2012) Brain derived neurotrophic factor inhibits apoptosis in enteric glia during gut inflammation. Med Sci Monit Int Med J Exp Clin Res 18: BR117–122. https://doi.org/10.12659/msm.882612
Ma D, Forsythe P, Bienenstock J (2004) Live Lactobacillus rhamnosus [corrected] is essential for the inhibitory effect on tumor necrosis factor alpha-induced interleukin-8 expression. Infect Immun 72: 5308–5314. https://doi.org/10.1128/IAI.72.9.5308-5314.2004
Ju IG, Hong SM, Yun S-W, Huh E, Kim D-H, Kim SY, Oh MS (2021) CCL01, a novel formulation composed of Cuscuta seeds and Lactobacillus paracasei NK112, enhances memory function via nerve growth factor-mediated neurogenesis. Food Funct 12: 10690–10699. https://doi.org/10.1039/d1fo01403j
Rahimlou M, Hosseini SA, Majdinasab N, Haghighizadeh MH, Husain D (2022) Effects of long-term administration of Multi-Strain Probiotic on circulating levels of BDNF, NGF, IL-6 and mental health in patients with multiple sclerosis: a randomized, double-blind, placebo-controlled trial. Nutr Neurosci 25: 411–422. https://doi.org/10.1080/1028415X.2020.1758887
Aygun H, Akin AT, Kızılaslan N, Sumbul O, Karabulut D (2022) Electrophysiological, histopathological and biochemical evaluation of the protective effect of probiotic supplementation against PTZ-induced seizures in rats. Eur J Neurol. https://doi.org/10.1111/ene.15359
Abenavoli L, Scarpellini E, Colica C, Boccuto L, Salehi B, Sharifi-rad J, Aiello V, Romano B, Lorenzo A De, Izzo AA (2019) Gut Microbiota and Obesity: A Role for Probiotics. Nutrients 11: 2690. https://doi.org/10.3390/nu11112690
Expert panel on detection evaluation and treatment of high blood cholesterol in adults (2001) Executive summary of the third report (NCEP) -adult treatment panel III. J Am Med Assoc 285: 2486–2497.
Wu X, Chen PS, Dallas S, Wilson B, Block ML, Wang CC, Kinyamu H, Lu N, Gao X, Leng Y, Chuang DM, Zhang W, Lu RB, Hong JS (2008) Histone deacetylase inhibitors up-regulate astrocyte GDNF and BDNF gene transcription and protect dopaminergic neurons. Int J Neuropsychopharmacol 11: 1123–1134. https://doi.org/10.1017/S1461145708009024
Spichak S, Donoso F, Moloney GM, Gunnigle E, Brown JM, Codagnone M, Dinan TG, Cryan JF (2021) Microbially-derived short-chain fatty acids impact astrocyte gene expression in a sex-specific manner. Brain, Behav Immun—Heal 16: 100318. https://doi.org/10.1016/j.bbih.2021.100318
Fukuchi M, Kirikoshi Y, Mori A, Eda R, Ihara D, Takasaki I, Tabuchi A, Tsuda M (2014) Excitatory GABA induces BDNF transcription via CRTC1 and phosphorylated CREB-related pathways in immature cortical cells. J Neurochem 131: 134–146. https://doi.org/10.1111/jnc.12801
Strandwitz P, Kim KH, Terekhova D, Liu JK, Sharma A, Levering J, McDonald D, Dietrich D, Ramadhar TR, Lekbua A, Mroue N, Liston C, Stewart EJ, Dubin MJ, Zengler K, Knight R, Gilbert JA, Clardy J, Lewis K (2019) GABA Modulating Bacteria of the Human Gut Microbiota. Nat Microbiol 4: 396–403. https://doi.org/10.1038/s41564-018-0307-3.GABA
Allam-Ndoul B, Castonguay-Paradis S, Veilleux A (2020) Gut microbiota and intestinal trans-epithelial permeability. Int J Mol Sci 21: 1–14. https://doi.org/10.3390/ijms21176402
Enzyme Database—BRENDA. https://www.brenda-enzymes.org/index.php. Accessed 26 Dec 2021
Yogeswara IBA, Maneerat S, Haltrich D (2020) Glutamate decarboxylase from lactic acid bacteria—a key enzyme in Gaba synthesis. Microorganisms 8: 1–24. https://doi.org/10.3390/microorganisms8121923
Louis P, Flint HJ (2017) Formation of propionate and butyrate by the human colonic microbiota. Env Microbiol 19: 29–41. https://doi.org/10.1111/1462-2920.
Corfield AP, Wagner SA, Clamp JR, Kriaris MS, Hoskins LC (1992) Mucin degradation in the human colon: production of sialidase, sialate O-acetylesterase, N-acetylneuraminate lyase, arylesterase, and glycosulfatase activities by strains of fecal bacteria. Infect Immun 60: 3971–3978. https://doi.org/10.1128/iai.60.10.3971-3978.1992
Wright DP, Rosendale DI, Roberton AM (2000) Prevotella enzymes involved in mucin oligosaccharide degradation and evidence for a small operon of genes expressed during growth on mucin. FEMS Microbiol Lett 190: 73–79. https://doi.org/10.1016/S0378-1097(00)00324-4
Chmilewsky F, About I, Chung SH (2017) C5L2 Receptor Represses Brain-Derived Neurotrophic Factor Secretion in Lipoteichoic Acid-Stimulated Pulp Fibroblasts. J Dent Res 96: 92–99. https://doi.org/10.1177/0022034516673832
West AE, Pruunsild P, Timmusk T (2014) Neurotrophic Factors: Transcription and Translation.
Murata K, Sawaji Y, Alimasi W, Suzuki H, Endo K, Tanaka H, Yorifuji M, Kosaka T, Shishido T, Yamamoto K (2016) PGE1 Attenuates IL-1β-induced NGF Expression in Human Intervertebral Disc Cells. Spine (Phila Pa 1976) 41: E710–E716. https://doi.org/10.1097/BRS.0000000000001379
Weigt SS, Palchevskiy V, Belperio JA (2017) Inflammasomes and IL-1 biology in the pathogenesis of allograft dysfunction. J Clin Invest 127: 2022–2029. https://doi.org/10.1172/JCI93537
Nagura N, Uchida K, Kenmoku T, Inoue G, Nakawaki M, Miyagi M, Takaso M (2019) IL-1β mediates NGF and COX-2 expression through transforming growth factor-activating kinase 1 in subacromial bursa cells derived from rotator cuff tear patients. J Orthop Sci 24: 925–929. https://doi.org/10.1016/j.jos.2019.02.006
Cox AJ, West NP, Cripps AW (2015) Obesity, inflammation, and the gut microbiota. Lancet Diabetes Endocrinol 3: 207–215. https://doi.org/10.1016/S2213-8587(14)70134-2
Rial SA, Karelis AD, Bergeron KF, Mounier C (2016) Gut microbiota and metabolic health: The potential beneficial effects of a medium chain triglyceride diet in obese individuals. Nutrients 8: 1–19. https://doi.org/10.3390/nu8050281
Funding
This work was performed under contract # 0373100122119000041 within the project “Creation of a bank of biosamples of blood serum and feces from healthy donors and patients with obesity, metabolic syndrome, type 2 diabetes mellitus, and impaired mucosal barrier of the gastrointestinal tract, in order to identify candidate species-nonspecific mediators of the quorum sensing microbiota systems of human, which modulate the endocrine and metabolic function of adipose tissue”.
Author information
Authors and Affiliations
Contributions
Conceptualization (A.M.G., S.A.R., V.V.M., S.M.Yu., A.V.Sh.); experimental design (A.M.G., S.A.R., N.I.V., T.V.G., V.V.M., S.M.Yu., A.V.Sh.); data collection (L.A.G., N.I.V., T.V.G., A.V.L.); data processing (I.M.K., L.A.G., N.I.V., T.V.G., A.V.L.); writing the manuscript (I.M.K., A.V.Sh.); editing the manuscript A.M.G., S.A.R., V.V.M., S.M.Yu., A.V.Sh.).
Corresponding author
Ethics declarations
COMPLIANCE WITH ETHICAL STANDARDS
All experimental procedures that involved human subjects complied with the ethical standards of the National Research Ethics Committee and the 1964 Declaration of Helsinki with its subsequent modifications or comparable ethical standards. The conduct of the research was approved by Local Ethics Committee of Pirogov Russian National Research Medical University (protocol no. 186 dated 06/26/2019) and Ethics Committee of Rostov State Medical University (protocol no. 20/19 dated 12/12/2019). A voluntary informed consent was obtained from each of the participants included in the study.
CONFLICT OF INTEREST
The authors declare that they have neither evident nor potential conflict of interest related to the publication of this article.
Additional information
Translated by A. Polyanovsky
Russian Text © The Author(s), 2022, published in Zhurnal Evolyutsionnoi Biokhimii i Fiziologii, 2022, Vol. 58, No. 4, pp. 298–310https://doi.org/10.31857/S0044452922040076.
Rights and permissions
About this article
Cite this article
Kolesnikova, I.M., Gaponov, A.M., Roumiantsev, S.A. et al. Relationship between Neutrophins and Gut Microbiome in Various Metabolic Types of Obesity. J Evol Biochem Phys 58, 986–1000 (2022). https://doi.org/10.1134/S0022093022040056
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022093022040056