Abstract
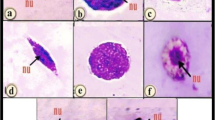
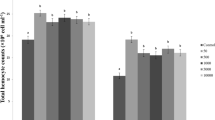
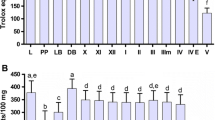
Activities of enzymatic antioxidants—superoxide dismutase, glutathione-S-transferase, and catalase—as well as generation of reactive oxygen species (ROS) in lymph of the honeycomb moth Galleria mellonella L. were studied at development of the process of encapsulation of nylon implants. It has been established that as soon as 15 min after piercing of cuticle with the implant the capsule is formed on its surface. Active melanization of the capsule has been shown to last for 4 h. A statistically significant increase of the ROS generation in lymph and a decrease of the enzymatic antioxidant activities in the insect hemocytes have been revealed after the implant incorporation. The authors suggest that the key role in maintenance of the oxidation-reduction balance in hemolymph at development of the encapsulation process is played by non-oxidative antioxidants.
Similar content being viewed by others
References
Ratcliffe, N.A. and Gagen, S.J., Studies on the in vivo Cellular Reactions of Insects: an Ultrastructural Analysis of Nodule Formation in Galleria mellonella, Tissue and Cell, 1977, vol. 9, pp. 73–85.
Lavine, M.D. and Strand, M.R., Insect Haemocytes and Their Role in Immunity, Insect Biochem. Mol. Biol., 2002, vol. 32, pp. 1295–1309.
Sugumaran, M., Comparative Biochemistry of Eumelanogenesis and the Protective Roles of Phenoloxidase and Melanin in Insects, Pigment Cell Res., 2002, vol. 15, pp. 2–9.
Slepneva, I.A., Komarov, D.A., Glupov, V.V., Serebrov, V.V., and Khramtsov, V.V., Influence of Fungal Infection on the DOPA-Semiquinone and DOPA-Quinone Production in Haemolymph ofGalleria mellonella Larvae, Biochem. Biophys. Res. Commun., 2003, vol. 300, pp. 188–191.
Komarov, D.A., Slepneva, I.A., Dubovskii, I.M., and Grizanova, E.V., Khramtsov, V.V., and Glupov, V.V., Generation of Superoxide Radical and Hydrogen Peroxide in Insect Hemolymph in the Course of Immune Response, Dokl. Biol. Sci., 2006, vol. 411, pp. 482–485.
Nappi, A.J. and Vass, E., Hydrogen Peroxide Production in Immune-Reactive Drosophila melanogaster, J. Parasitol., 1998, vol. 84, pp. 1150–1157.
Nappi, A.J., Vass, E., Frey, F., and Carton, Y., Superoxide Anion Generation in Drosophila during Melanotic Encapsulation of Parasites, Eur. J. Cell Biol., 1995, vol. 68, pp. 450–456.
Whitten, M.M.A. and Ratcliffe, N.A.,In vitro Superoxide Activity in the Haemolymph of the West Indian Leaf Cockroach. Blaberus discoidalis, J. Insect Physiol., 1999, vol. 45, pp. 667–675.
Nappi, A.J., Vass, E., Frey, F., and Carton, Y., Nitric Oxide Involvement in Drosophila Immunity, Nitric Oxide, 2000, vol. 4, pp. 423–430.
Glupov, V.V., Slepneva, I.A., Serebrov, V.V., Khvoschevskaya, M.F., Martem’yanov, V.V., Dubovskiy, I.M., and Khramtsov, V.V., Influence of the Fungal Infection on the Production of Reactive Metabolites and the Antioxidant State of Haemolymph of Galleria mellonella Larvae, Russ. Entomol. J., 2003, vol. 12, pp. 103–108.
Lozinskaya, Ya.L., Slepneva, I.A., Khramtsov, V.V., and Glupov, V.V., Change of Antioxidant Status and of System Generation of Free Radicals in Hemolymph of Larvae Galleria mellonella in Microsporidiosis, Zh. Evol. Biokhim. Fiziol., 2004, vol. 40, pp. 99–103.
Lanaz-Mendoza, H., Hernandez-Martinez, S., Ku-Lopez, M., Rodriguez, M.D., Herrera-Ortiz, A., and Rodriguez, M.H., Superoxide Anion in Anopheles albimanus Hemolymph and Midgut Is Toxic to Plasmodium berghei Ookinetes, J. Parasitol., 2002, vol. 88, pp. 702–706.
Kumar, S., Cristophides, G.K., Cantera, R., Charles, B., Han, Y.S., Meister, S., Dimopoulos, G., Kafatos, F.C., and Barillas-Mury, C., The Role of Reactive Oxygen Species in Plasmodium melanotic Encapsulation on Anopheles gambiae, Proc. Nat. Acad. Sci. USA, 2003, vol. 100, pp. 14139–14144.
Nappi, A.J. and Christensen, B.M., Melanogenesis and Associated Cytotoxic Reactions: Applications to Insect Innate Immunity, Insect Biochem. Mol. Biol., 2005, vol. 35, pp. 443–459.
Glupov, V.V., Khvoshevskaya, M.F., Lozinskaya, Ya.L., Dubovskii, I.M., Martem’yanov, V.V., and Sokolova, Ju.Ya., Application of the Method NBT-Reduction for Studies on the Production of Reactive Oxigen Species in Insect Haemocytes, Cytobios, 2001, vol. 106, pp. 165–178.
Carton, Y., Poiri’e, M., and Nappi, A.J., Insect Immune Resistance to Parasitoids, Insect Sci., 2008, vol. 15, pp. 67–87.
Zenkov, N.K., Lankin, V.Z., and Menshchikova, E.B., Okislitel’nyi stress: Biokhimicheskii i patofiziologicheskii aspekty (Oxidative Stress: Biochemical and Pathophysiological Aspects), Moscow, 2001.
Felton, G.W. and Summers, C.B., Antioxidant Systems in Insects, Arch. Insect Biochem. Physiol., 1995, vol. 2, pp. 187–197.
Wang, Y., Oberley, L.W., and Murhammer, D.W., Antioxidant Defense Systems of Two Lepidopteran Insect Cell Lines, Free Radical Biol. Med., 2001, vol. 30, pp. 1254–1262.
Barbehenn, R.V., Gut-Based Antioxidant Enzymes in a Polyphagous and a Graminivorous Grasshopper, J. Chem. Ecol., 2002, vol. 28, pp. 1329–1347.
Mastore, M., Kohler, L., and Nappi, A.J., Production and Utilization of Hydrogen Peroxide Associated with Melanogenesis and Tyrosinase-Mediated Oxidations of DOPA and Dopamine, Free Radical Res. 2005, vol. 39, pp. 853–858.
Winterbourn, C.C., Pichorner, H., and Kettle, A.J., Myeloperoxidase-Dependent Generation of a Tyrosine Peroxide by Neutrophils, Arch. Biochem. Biophys., 1997, vol. 338, pp. 15–21.
Rantala, M.J. and Roff, D.A., Analysis of the Importance of Genotypic Variation, Metabolic Rate, Morphology, Sex and Development Time on Immune Function in the Cricket, Gryllus firmus, J. Evol. Biol., 2006, vol. 19, pp. 834–843.
Dubovskii, I.M., Krukova, N.A., and Glupov, V.V., Phagocytic Activity and Encapsulation Rate of Galleria mellonella Larvae Hemocytes during Bacterial Infection by Bacillus thu-ringiensis, J. Invertebr. Pathol., 2008, vol. 98, no. 3, pp. 360–362.
McCord, J.M. and Fridocich, I., Superoxide Dismutase: an Enzymic Function for Erythrocuprein (Hemocuprein), J. Biol. Chem., 1969, vol. 244, pp. 6049–6055.
Habig, W.H., Pabst, M.J., and Jakoby, W.B., Glutathione-S-Transferases, J. Biol. Chem., 1974, vol. 249, pp. 7130–7139.
Wang, Y., Oberley, L.W., and Murhammer, D.W., Evidence of Oxidative Stress Following the Viral Infection of Two Lepidopteran Insect Cell Lines, Free Radical Biol. Med., 2001, vol. 31, pp. 1448–1455.
Dijalov, S., Skatchkov, M., and Bassenge, E., Spin Trapping of Superoxide Radicals and Peroxynitrite by 1-Hydroxy-3-carboxy-pyrrolidine and 1-Hydroxy-2,2,6,6-tetramethyl-4-oxopiperidine and the Stability of Corresponding Nitroxyl Radicals towards Biological Reductants, Biochem. Biophys. Res. Commun., 1997, vol. 320, pp. 230–237.
Jiang, Z.-Y., Woollard, A.C.S., and Wolff, S.P., Hydrogen Peroxide Production during Experimental Protein Glycation, FEBS Lett., 1990, vol. 268, pp. 69–71.
Bradford, M.M., A Rapid And Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein Dye Binding, Anal. Biochem., 1976, vol. 72, pp. 248–254.
Ling, E. and Yi, X.Q., Prophenoloxidase Binds to the Surface of Hemocytes and Is Involved in Hemocyte Melanization in Manduca sexta, Insect Biochem. Mol. Biol., 2005, vol. 35, pp. 1356–1366.
Slepneva, I.A., Glupov, V.V., Sergeeva, S.V., and Khramtsov, V.V., EPR Detection of Reactive Oxygen Species in Hemolymph ofGalleria mellonella and Dendrolimus supe-rans sibiricus (Lepidoptera) Larvae, Biochem. Biophys. Res. Commun., 1999, vol. 264, pp. 212–215.
Oreste, A. and De Tullio, M.C., Ascorbic Acid: Much More Than Just an Antioxidant, Biochim. Biophys. Acta, 2002, vol. 1569, pp. 1–9.
Krishnan, N., Hyrš, P., and Šimek, V., Nitric Oxide Production by Hemocytes of Larva and Pharate Prepupa of Galleria mellonella in Response to Bacterial Lipopolysaccharide: Cytoprotective or Cytotoxic?, Comp. Biochem. Physiol., Part C, 2006, vol. 142, pp. 103–110.
Stoian, I., Oros, A., and Moldoveanu, E., Apoptosis and Free Radicals, Biochem. Mol. Med., 1996, vol. 59, pp. 93–97.
Author information
Authors and Affiliations
Additional information
Original Russian Text © I. M. Dubovskii, E. V. Grizanova, E. A. Chertkova, I. A. Slepneva, D. A. Komarov, Ya. L. Vorontsova, V. V. Glupov, 2010, published in Zhurnal Evolyutsionnoi Biokhimii i Fiziologii, 2010, Vol. 46, No. 1, pp. 30–36.
Rights and permissions
About this article
Cite this article
Dubovskii, I.M., Grizanova, E.V., Chertkova, E.A. et al. Generation of reactive oxygen species and activity of antioxidants in hemolymph of the moth larvae Galleria mellonella (L.) (Lepidoptera: Piralidae) at development of the process of encapsulation. J Evol Biochem Phys 46, 35–43 (2010). https://doi.org/10.1134/S0022093010010044
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022093010010044