Abstract
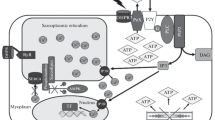
Based on the earlier discovered by the authors adenylyl cyclase signaling mechanisms (ACSM) of action of insulin and relaxin, a study was performed of the existence of a similar action mechanism of another representative of the insulin superfamily-the insulin—like growth factor 1 (IGF-1) in the muscle tissue of vertebrates (rat) and invertebrates (mollusc). For the first time there was detected participation of ACSM in the IGF-1 action, including the six-component signaling cascade: receptor tyrosine kinase → Gi-protein (βγ-dimer) → phosphatidylinositol-3-kinase (PI-3K) → protein kinase Cζ (PKCζ) → Gs-protein → adenylyl cyclase. By structural-functional organization at postreceptor stages, it coincides completely with that of insulin and relaxin, which we revealed in rat skeletal muscle. In smooth muscle of the mollusc Anodonta cygnea this ACSM of action of IGF-1 has only one difference-the protein kinase C included in this mechanism is represented not by the PKCζ isoform, but by another isoform close to PKCε of the vertebrate brain. Earlier we revealed the same differences in muscles of this mollusc in the ACSM of action of insulin and relaxin.
Similar content being viewed by others
References
Steiner, D.F., Chan, S.J., Docherty, K., et al., Evolution of Polypeptide Hormones and Their Precursor Processing Mechanism, Evolution and Tumor Pathology of the Neuroendocrine System, Falkmer, S., Hakanson, R., and Sandler, F., Eds., vol. 12, Elsevier Science Publishers BV, 1984, pp. 203–223.
Chun, S.J., Nagamatsu, S., Cao, Q.P., and Steiner, D.F., Structure and Evolution of Insulin and Insulin-Like Growth Factors in Chordates, Progress in Brain Research, Joosse, J., Buijs, R.M., and Tilders, F.J.H., Eds., vol. 92, Elsevier Science Publishers BV, 1992, pp. 15–24.
Murray-Rust, J., Macleod, A.N., Blundell, T.L., and Wood, S.P., Structure and Evolution of Insulins: Implifications for Receptor Binding, BioEssays, 1992, vol. 14, pp. 325–331.
Pertseva, M.N., Shpakov, A.O., Plesneva, S.A., and Kuznetsova, L.A., A Novel View on the Mechanisms of Action of Insulin and Other Insulin Supcrfamily Peptides: Involvement of Adenylyl Cyclase Signaling System, Comp. Biochem. Physiol., 2003, vol. 134, pp. 11–36.
Patel, T.B., Signal Transmembrane Spanning Heterotrimeric G-Protein Coupled Receptors and Their Signaling Cascades, Pharmacol. Rev., 2004, vol. 56, pp. 371–385.
Plesneva, S.A., Shpakov, A.O., Rusakov, Yu.I., Kuznetsova, L.A., and Pertseva, M.N., The Involvement of Adenylate Cyclase Signaling System in the Action of Insulin, Epidermal Growth Factor and Insulin-Like Peptide of Mollusc, Proceedings of 1th Conference of European Comparative Endocrinologists, Spain (September 5–10, 1994), Roubos, W.W., Ed., Universiteit Cordoba, Abstract 135, Cordoba: Univ., 1994.
Pertseva, M.N., Plesneva, S.A., Shpakov, A.O., Rusakov, Yu.I., and Kuznetsova, L.A., Involvement of Adenylyl Cyclase Signaling System in the Action of Insulin and Mollusc Insulin-Like Peptide, Comp. Biochem. Physiol., 1995, vol. 112, pp. 689–695.
Pertseva, M.N., Shpakov, A.O., and Plesneva, S.A., Current Advances in Studies of Signaling Mechanisms of Action of Insulin and Related Peptides, J. Evol. Biochem. Physiol., 1996, vol. 32, pp. 259–277.
Pertseva, M.N., Plesneva, S.A., Kuznetsova, L.A., Shpakov, A.O., and Derkach, K.V., On the Tyrosine Kinase Mechanism of the Novel Effect of Insulin and Insulin-Like Growth Factor 1. Stimulation of the Adenylyl Cyclase System in Muscle Tissues, Biochem. Pharmacol., 1996, vol. 52, pp. 1867–1874.
Plesneva, S.A., Pospelova, T.V., Kuznetsova, L.A., Bykova, T.V., Shpakov, A.O., and Pertseva, M.N., A Novel Insulin-Competent Adenylyl Cyclase Signaling System as a Possible Mechanism of Antiapoptotic Action of Insulin and Insulin-Like Growth Factor 1, Dokl. Akad. Nauk, 2003, vol. 393, pp. 551–553.
Plesneva, S.A., Shpakov, A.O., Kuznetsova, L.A., and Pertseva, M.N., A Dual Role of Protein Kinase C in Insulin Signal Transduction via Adenylyl Cyclase Signaling System in Muscle Tissues of Vertebrates and Invertebrates, Biochem. Pharmacol., 2001, vol. 61, pp. 1277–1291.
Pertseva, M.N., Shpakov, A.O., Kuznetsova, L.A., and Omeljaniuk, E., Adenylyl Cyclase Signaling Mechanisms of Insulin and Relaxin Action: Similarities and Differences, Gen. Biol. Int., 2006, vol. 30, pp. 533–540.
Shpakov, A.O., Shipilov, V.N., Bondareva, V.M., Kuznetsova, L.A., Plesneva, S.A., Rusakov, Yu.I., and Pertseva, M.N., Regulating Action of InsulinRelated Neuropeptides of Mollusc Anodonta cygnea on Functional Activity of Adenylyl Cyclase Signaling System, Neirokhimiya, 2005, vol. 22, no. 1, pp. 28–37.
Butler, A.A., Yakar, S., Gewolb, I.H., Karas, M., Okubo, Y., and Le Roith, D., Insulin-Like Growth Factor-1 Receptor Signal Transduction: At the Interface between Physiology and Cell Biology, Comp. Biochem. Physiol., 1992, vol. 121, pp. 19–26.
Hallak, H., Seiler, A.E., Green, J.S., Ross, B.N., and Rubin, R., Association of Heterotrimeric G(i) with Insulin-Like Growth Factor-1 Receptor. Release of G(beta-gamma) Subunits upon Receptor Activation, J. Biol. Chem., 2000, vol. 275, pp. 2255–2258.
Dalle, S., Imamura, T., Vollenwieder, P., and Olefsky, J.M., Insulin and Insulin-Like Growth Factor I Receptors Utilize Different G Protein Signaling Components, J. Biol. Chem., 2001, vol. 276, pp. 15 688–15 695.
Kuemmerle, J.F. and Murthy, K.S., Coupling of the IGF-1 Receptor Tyrosine Kinase to Gi2 in Human Intestinal Smooth Muscle: Gβγ-Dependent MAP Kinase Activation and Growth, J. Biol. Chem., 2001, vol. 276, pp. 7187–7194.
Kidwai, A.M., Radcliffe, M.A., Lee, F.Y., and Daniel, E.E., Isolation and Properties of Skeletal Muscle Membranes, Biochim. Biophys. Acta, 1973, vol. 298, pp. 593–607.
Salomon, J., Londos, C., and Rodbell, M., A Highly Sensitive Adenylate Cyclase Assay, Anal. Biochem., 1974, vol. 58, pp. 541–548.
Milligan, G., Techniques Used in the Identification and Analysis of Function of Pertussis ToxinSensitive Guanine Nucleotide Binding Proteins, Biochem. J., 1988, vol. 255, pp. 1–13.
Reisine, T., Pertussis Toxin in the Analysis of Receptor Mechanisms, Biochem. Pharmacol., 1990, vol. 39, pp. 1499–1504.
Sossin, W.S., Chen, C.S., and Toker, A., Stimulation of the Insulin Receptor Activates and Downregulates the Ca2+-Independent Protein Kinase C, Apl-II through a Wortmannin-Sensitive Signaling Pathway in Aplysia, J. Neurochem., 1996, vol. 67, pp. 220–228.
Mercado, L., Cao, A., Barcia, R., and RamosMartinez, J.I., Regulatory Properties of p105: A Novel PKC Isoenzyme in Mantle Tissues from Marine Mussels, Biochem. Cell Biol., 2002, vol. 80, pp. 771–775.
Okamoto, J., Okamoto, T., Murayama, J., Hayashi, J., Ogata, E., and Nashimoto, I., GTP-Binding Protein Activator Sequences in the Insulin Receptor, FEBS Lett., 1993, vol. 334, pp. 143–148.
Shpakov, A.O., Homology of Primary Structure of the Third Cytoplasmic Domains of Receptors of the Rhodopsin Type and Cytoplasmic Tail of β-Subunits of Insulin Receptor, Tsitologiya, 1996, vol. 38, pp. 1179–1190.
Pertseva, M.N., Hypothesis about the Key Coordinating Role of the Adenylyl Cyclase Signaling Mechanism and cAMP in Regulatory Action of Peptides of the Insulin Superfamily on Fundamental Cellular Processes: Cellular Growth, Apoptosis, Metabolism, Zh. Evol. Biokhim. Fiziol., 2000, vol. 36, pp. 496–503.
Plesneva, S.A., Barkan, R.S., Reshetnikova, G.F., Kuznetsova, L.A., and Pertseva, M.N., Adenylyl Cyclase Signaling Mechanism in Mitogenic Action of Insulin, the Insulin-Like and Epidermal Growth Factors, Dokl. Akad. Nauk, 1999, vol. 368, pp. 833–838.
Kooijman, R., Regulation of Apoptosis by InsulinLike Growth Factor I (IGFI), Cytokine Growth Factor, 2006, vol. 17, pp. 305–323.
Author information
Authors and Affiliations
Additional information
Original Russian Text © S. A. Plesneva, L. A. Kuznetsova, A. O. Shpakov, T. S. Sharova, M. N. Pertseva, 2008, published in Zhurnal Evolyutsionnoi Biokhimii i Fiziologii, 2008, Vol. 44, No. 5, pp. 459–466.
Rights and permissions
About this article
Cite this article
Plesneva, S.A., Kuznetsova, L.A., Shpakov, A.O. et al. Study of structural-functional arrangement of the adenylyl cyclase signaling mechanism of action of insulin-like growth factor 1 revealed in muscle tissue of representatives of vertebrates and invertebrates. J Evol Biochem Phys 44, 542–551 (2008). https://doi.org/10.1134/S0022093008050022
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022093008050022