Abstract
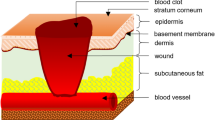
Normal course of processes of regeneration and epithelization of damaged tissues has been shown to be based on the capability of cells participating in these processes for selective adhesion. In the case of the complete or partial absence of this capability in the cells-participants of the wound healing process, the so-called non-healing wounds appear. In this connection, it remains actual to search for natural agents promoting healing of chronic non-healing wounds. In the present work, we studied effects of synthetic fragments of leukocytic antimicrobial peptides defensines—GER, FGER, and GERA—on aggregation and adhesion of epitheliolike cells of the CHO-K1 line. These peptides have been established to have aggregate-stimulating properties; besides, they enhance adhesion of the cells to the untreated plastic and inhibit fibronectinmediated cell adhesion. Possible pathways of regulation by peptides of processes of intercellular and cell-matrix interaction are discussed as well as ways of release of these compounds in an organism and their functional role in an organism.
Similar content being viewed by others
References
Fenchin, K.M., Zazhivlenie ran (Wound Healing), Kiev, 1979.
Kokryakov, B.N., Biologiya antibiotikov zhivotnogo proiskhozhdeniya (Biology of Antibiotics of the Animal Origin), St. Petersburg, 1999.
Sorensen, O.E., Cowland, J.B., Theilgaard-Monch, K., Liu, L., Gans, T., and Borregaard, N., Wound Healing and Expression of Antimicrobial Peptides/Polypeptides in Human Keratinocytes, a Consequence of Common Growth Factor, J. Immunol., 2003, vol. 170, pp. 5583–5589.
Kirsner, R.S. and Eaglstein, W.H., The Wound Healing Process, Dermatol. Clin., 1993, pp. 629–640.
Ivanova, V.P., Sorochinskaya, E.I., Lozhkina, T.K., and Anokhina, V.V., Immunomodulating and Analgesic Activity of Synthetic Fragments of Various Proteins and Immunopeptides, Ukr. Biokhim. Zh., 1988, vol. 60, pp. 3–9.
Bromberg, J.S., The Biology of CD2: Adhesion, Transmembrane Signal and Regulatory Receptor of Immunity, J. Surg. Res., 1993, vol. 54, pp. 258–267.
Bierer, B.E. and Hahn, W.C., T Cell Adhesion, Avidity Regulation and Signaling: a Molecular Analysis of CD2, Semin. Immunol., 1993, vol. 5, pp. 249–261.
Takai, Y., Reed, M.L., Burakoff, S.J., and Hermann, S.H., Direct Evidence for a Receptor-Ligand Interaction between the T Cell Surface Antigen CD2 and Lymphocyte-Function-Associated Antigen 3, Proc. Natl. Acad. Sci. USA, 1987, vol. 84, pp. 6864–6868.
Rechy, M.A., Neidhardt, E.A., Sayre, P.H., Ciardelli, T.L., and Reinherz, E.L., Structural and Functional Characterization of the CD2 Immunoadhesion Domain. Evidence for Inclusion of CD2 in an Alpha-Beta Protein Folding Class, J. Biol. Chem., 1990, vol. 265, pp. 8542–8549.
Rabin, E.M., Gordon, K., Knoppers, M.N., Luther, M.A., Neidhardt, E.A., Flynn, J.F., Sardonini, C.A., Sampo, T.M., Cancino, M.F., and Recny, M.A., Inhibition of T Cell Activation and Adhesion Functions by Soluble CD2 Protein, Cell Immunol., 1993, vol. 149, pp. 24–38.
Yukuva, N., Ivone, T., and Tadashi, E.A., A Novel Neutrophil Adherence Test Effectively Reflects the Activated State of Neutrophils, Microbiol. Immunol., 1989. vol. 33, pp. 834–852.
Paltsev, M.A., Ivanov, A.A., and Severin, S.E., Mezhkletochnye vzaimodeistviya (Intercellular Interactions), Moscow, 2003.
Komberger, D.J., Fibronectin, Int. J. Bjochem. Cell. Biol., 1997, vol. 29, pp. 939–943.
Humphries, M.J., Integrin Structure, Biochem. Soc. Trans., 2000, vol. 28, pp. 311–339.
van der Flier, A. and Sonnenberg, A., Function and Interactions of Integrins, Cell Tissue Res., 2001, vol. 305, pp. 285–298.
Johansson, S., Svineng, G., Wennerberg, K., Armutik, A., and Lohikangas, L., Fibronectin-Integrin Interactions, Front. Biosci., 1997, vol. 2, pp. 126–146.
Miyamoto, S., Teramoto, H., Coso, O.A., Gutkind, J.S., Burbelo, P.D., Akiyama, S.K., and Yamada, K.M., Integrin Function: Molecular Hierarchies of Cytosceletal and Signaling Molecules, J. Cell Biol., 1995, vol. 131, pp. 791–805.
Loo, D.T., Kanner, S.B., and Aruffo, A., Filamin Binds to the Cytoplasmic Domain of the Beta 1-Integrin. Identification of Amino Acids Responsible for this Interaction, J. Biol. Chem., 1998, vol. 273, pp. 23 304–23 312.
Otey, C.A., Vasquer, G.B., Burridge, K., and Erickson, B.W., Mapping of the α-Actinin Binding Site within the β1-Integrin Cytoplasmic Domain, J. Biol. Chem., 1993, vol. 268, pp. 21 193–21 197.
Reddy, K.B., Gascard, P., Price, M.G., Negrescu, E.V., and Fox, J.E.B., Identification of an Interaction between the M-Band Protein Skelemin and β-Integrin Subunits. Colocalisation of a Skelemin-Like Protein with β1-and β3-Integrins in Nonmuscle Cells, J. Biol. Chem., 1998, vol. 273, pp. 35 039–35 047.
Chen, L.M., Bailey, D., and Fernandez-Valle, C., Association of β1 Integrin with Focal Adhesion Kinase and Paxillin in Defferentiating Schwann Cells, J. Neurosci., 2000, vol. 20, pp. 3776–3784.
Knight, C.G., Morton, L.F., Onley, D.J., Peachey, A.R., Messent, A.J., Smethurst, P.A., Tuckwell, D.S., Farndale, R.W., and Barnes, M.J., Identification in Collagen Type I of an Integrin α2β1-Binding Site Containing an Essential GER Sequence, J. Biol. Chem., 1998, vol. 273, pp. 33 287–33 294.
Xu, Y., Gurusiddappa, S., Rich, R.L., Owens, R.T., Keene, D.R., Mayne, R., Hook, A., and Hook, M., Multiple Binding Sites in Collagen Type I for the Integrins α1β1 and α2β1, J. Biol. Chem., 2000, vol. 275, pp. 38 981–38 989.
Gullberg, D.E. and Lundgren-Akerlund, E., Collagen-Binding I Domain Integrins—What Do They Do?, Prog. Histochem. Cytochem., 2002, vol. 37, pp. 3–54.
Soo, C., Shaw, W.W., Zhang, X., Longaker, M.T., Howard, E.W., and Ting, K., Differential Expression of Matrix Metalloproteases and Their Tissue-Derived Inhibitors in Cutaneous Wound Repair, Plast. Reconstr. Surg., 2000, vol. 105, pp. 638–647.
Kharchenko, E.P., Ivanova, V.P., Sokolova, T.V., and Levchenko, V.F., The Block Principle of Organization and Polyfunctionality of Regulatory Peptides, Biokhimiya, 1987, vol. 52, pp. 279–289.
Author information
Authors and Affiliations
Additional information
Original Russian Text © V. P. Ivanova, Z. V. Kovaleva, E. I. Sorochinskaya, V. V. Anokhina, and A.I. Krivchenko, 2007, published in Zhurnal Evolyutsionnoi Biokhimii i Fiziologii, 2007, Vol. 43, No. 4, pp. 337–345.
Rights and permissions
About this article
Cite this article
Ivanova, V.P., Kovaleva, Z.V., Sorochinskaya, E.I. et al. Effects of synthetic defensin fragments on aggregation and adhesion of epitheliolike cells. J Evol Biochem Phys 43, 404–414 (2007). https://doi.org/10.1134/S0022093007030059
Received:
Issue Date:
DOI: https://doi.org/10.1134/S0022093007030059