Abstract—
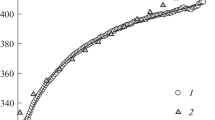
The CaSm2Ge3O10 germanate has been prepared by firing stoichiometric mixtures of CaCO3, Sm2O3, and GeO2 in air in the temperature range 1423–1473 K. X-ray powder diffraction characterization with the use of the derivative difference minimization method has shown that CaSm2Ge3O10 has a monoclinic structure (sp. gr. P21/c, 293 К) with unit-cell parameters a = 6.9779(8) Å, b = 6.92859(7) Å, c = 18.8907(2) Å, and β = 108.3280(8)°. The high-temperature heat capacity of calcium samarium germanate samples has been determined in the temperature range 320–1000 K by differential scanning calorimetry and the experimental Cp(T) data have been used to evaluate thermodynamic properties of CaSm2Ge3O10.


Similar content being viewed by others
REFERENCES
Yamane, H., Tanimura, R., Yamada, T., et al., Synthesis and crystal structure of CaY2Ge3O10 and CaY2Ge4O12, J. Solid State Chem., 2006, vol. 179, pp. 289–295. https://doi.org/10.1016/j.jss.2005.10.023
Lipina, O.A., Surat, L.L., Melkozerova, M.A., et al., Synthesis, crystal structure and luminescence properties of CaY2–xEuxGe3O10 (x = 0–2), J. Solid State Chem., 2013, vol. 206, pp. 117–121. https://doi.org/10.1016/j.jssc.2013.08.002
Lipina, O.A., Surat, L.L., Melkozerova, M.A., et al., Synthesis, crystal structure, and luminescence properties of CaY2Ge3O10:Ln3+, Ln = Eu, Tb, Opt. Spectrosc., 2014, vol. 116, no. 5, pp. 695–699. https://doi.org/10.1134/S0030400X14050130
Lipina, O.A., Surat, L.L., Tyutyunnik, A.P., et al., Synthesis and structural study of a new group of trigermanates, CaRE2Ge3O10 (RE = La–Yb), CrystEngComm, 2015, pp. 1–12. https://doi.org/10.1039/c5ce00063g
Lipina, O.A., Surat, L.L., Tyutyunnik, A.P., et al., Infrared luminescence of CaLa2–xNdxGe3O10: Ho3+,Er3+, Opt. Spectrosc., 2016, vol. 121, no. 4, pp. 511–517. https://doi.org/10.1134/S0030400X1610012X
Lipina, O.A., Surat, L.L., Baklanova, Ya.V., et al., Thermal expansion and luminescent properties of triorthogermanates CaLa2–xEuxGe3O10 (x = 0.0–0.6), Phys. Solid State, 2018, vol. 60, no. 2, pp. 370–375. https://doi.org/10.1134/S1063783418020154
Lipina, O.A., Surat, L.L., Chufarov, A.Y., et al., Upconversion luminescence and radiometric temperature sensing behavior of Er3+/Yb3+-Codoped CaY2Ge3O10 germanate, Mendeleev Commun., 2021, vol. 31, no. 1, pp. 113–151. https://doi.org/10.1016/j.mencom.2021.01.035
Lipina, O.A., Surat, L.L., Melentsova, A.A., et al., BaYb2–xErxGe3O10 and BaY2–10yYb9yEryGe3O10: luminescent properties and prospects for applications in remote temperature determination, Phys. Solid State, 2021, vol. 63, no. 7, pp. 1036–1041. https://doi.org/10.1134/S1063783421070143
Visser, J.W., A fully automatic program for finding the unit cell from powder data, J. Appl. Crystallogr., 1969, vol. 2, pp. 89–95.
Solovyov, L.A., Full-profile refinement by derivative difference minimization, J. Appl. Crystallogr., 2004, vol. 37, pp. 743–749. https://doi.org/10.1107/S0021889804015638
Denisova, L.T., Irtyugo, L.A., Kargin, Yu.F., Beletskii, V.V., and Denisov, V.M., High-temperature heat capacity and thermodynamic properties of Tb2Sn2O7, Inorg. Mater., 2017, vol. 53, no. 1, pp. 93–95. https://doi.org/10.1134/S0020168517010046
Denisova, L.T., Kargin, Yu.F., and Denisov, V.M., Heat capacity of rare-earth stannates in the range 350–1000 K, Inorg. Mater., 2017, vol. 53, no. 9, pp. 956–961. https://doi.org/10.1134/S0020168517090059
Maier, C.G. and Kelley, K.K., An equation for the representation of high temperature heat content data, J. Am. Chem. Soc., 1932, vol. 54, no. 8, pp. 3243–3246. https://doi.org/10.1021/ja01347a029
Denisova, L.T., Irtyugo, L.A., Kargin, Yu.F., et al., Synthesis and high-temperature heat capacity of Sm2Ge2O7 and Eu2Ge2O7, Inorg. Mater., 2018, vol. 54, no. 2, pp. 167–170. https://doi.org/10.1134/S0020168518020048
Leitner, J., Chuchvalec, P., Sedmidubský, D., et al., Estimation of heat capacities of solid mixed oxides, Thermochim. Acta, 2003, vol. 395, pp. 27–46. https://doi.org/10.1016/S0040-6031(02)00176-6
Leitner, J., Voňka, P., Sedmidubský, D., and Svoboda, P., Application of Neumann–Kopp rule for estimation of heat capacity of mixed oxides, Thermochim. Acta, 2010, vol. 497, pp. 7–13. https://doi.org/10.1016/j.tca.2009.08.002
Kumok, V.N., Problem of matching techniques for evaluating thermodynamic characteristics, in Pryamye i obratnye zadachi khimicheskoi termodinamiki (Direct and Inverse Problems in Chemical Thermodynamics), Novosibirsk: Nauka, 1987, pp. 108–123.
Kubaschewski, O. and Alcock, S.B., Metallurgical Thermochemistry, Oxford: Pergamon, 1979, 5th ed.
Mostafa, A.T.M.G., Eakman, J.M., Montoya, M.M., and Yarbra, S.L., Prediction of heat capacities of solid inorganic salts from group contributions, Ind. Eng. Chem. Res., 1996, vol. 35, pp. 343–348.
Leitner, J., Sedmidubský, D., and Chuchvalec, P., Prediction of heat capacities of solid binary oxides from group contribution method, Ceram.-Silik., 2002, vol. 46, no. 1, pp. 29–32.
Tret’yakov, Yu.D., Tverdofaznye reaktsii (Solid-State Reactions), Moscow: Khimiya, 1978.
Zhang, Y. and Jung, I.-H., Critical evaluation of thermodynamic properties of rare earth sesquioxides (RE = La, Ce, Pr, Nd, Pm, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb, Lu, Sc and Y), CALPHAD: Comp. Coupling Phase Diagr. Thermochem., 2017, vol. 58, pp. 169–203. https://doi.org/10.1016/j.calphad.2017.07.001
Tananaev, I.V. and Shpirt, M.Ya., Khimiya germaniya (The Chemistry of Germanium), Moscow: Khimiya, 1967.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by O. Tsarev
Rights and permissions
About this article
Cite this article
Denisova, L.T., Galiakhmetova, N.A., Kargin, Y.F. et al. Synthesis, Crystal Structure, and Thermodynamic Properties of the CaSm2Ge3O10 Germanate in the Range 320–1000 K. Inorg Mater 59, 93–97 (2023). https://doi.org/10.1134/S0020168523010065
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0020168523010065