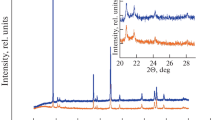
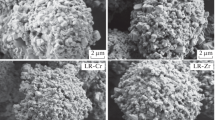
Abstract—
We have studied the effect of doping with tin and titanium cations on the electrochemical performance of lithium-rich cathode materials. Samples for this investigation were prepared via coprecipitation of precursors, followed by solid-state reaction with a lithium and tin (titanium) source. The cathode materials have been characterized by X-ray diffraction, scanning electron microscopy, and X-ray microanalysis and tested in lithium half-cells in galvanostatic cycling mode at various current densities. The titanium-doped material had a considerably higher specific discharge capacity (270 mAh/g) in comparison with the undoped and tin-doped materials (230 mAh/g). As the charge/discharge current was raised, the titanium-doped sample exhibited the best cycling stability among all of the materials. In addition, both doped materials had a smaller voltage hysteresis in comparison with the undoped sample.







Similar content being viewed by others
REFERENCES
Ramesha, R.N., Bosubabu, D., Kartick Babu, M.G., and Ramesha, K., Tuning of Ni, Mn, and Co (NMC) content in 0.4(LiNixMnyCozO2)·0.4(Li2MnO3) toward stable high-capacity lithium-rich cathode materials, ACS Appl. Energy Mater., 2020, vol. 3, pp. 10872–10881. https://doi.org/10.1021/acsaem.0c01897
Croy, J.R., Gallagher, K.G., Balasubramanian, M., Long, B.R., and Thackeray, M.M., Quantifying hysteresis and voltage fade in xLi2MnO3·(1 – x)LiMn0.5Ni0.5O2 electrodes as a function of Li2MnO3 content, J. Electrochem. Soc., 2014, vol. 161, pp. A318–A325. https://doi.org/10.1149/2.049403jes
Makhonina, E.V., Maslennikova, L.S., Volkov, V.V., Medvedeva, A.E., Rumyantsev, A.M., Koshtyal, Yu.M., Maximov, M.Yu., Pervov, V.S., and Eremenko, I.L., Li-rich and Ni-rich transition metal oxides: coating and core–shell structures, Appl. Surf. Sci., 2019, vol. 474, pp. 25–33. https://doi.org/10.1016/j.apsusc.2018.07.159
Ji, X., Xia, Q., Xu, Y., Feng, H., Wang, P., and Tan, Q., A review on progress of lithium-rich manganese-based cathodes for lithium ion batteries, J. Power Sources, 2021, vol. 487, p. 29362. https://doi.org/10.1016/j.jpowsour.2020.229362
Ates, M.N., Mukerjee, S., and Abraham, K.M., A search for the optimum lithium rich layered metal oxide cathode material for Li-ion batteries, J. Electrochem. Soc., 2015, vol. 162, pp. A1236–A1245. https://doi.org/10.1149/2.0481507jes
Viji, M., Budumuru, A.K., Hebbar, V., Gautam, S., Chae, K.H., and Sudakar, C., Influence of morphology and compositional mixing on the electrochemical performance of Li-rich layered oxides derived from nanoplatelet-shaped transition metal oxide–hydroxide precursors, Energy Fuels, 2021, vol. 35, pp. 4533–4549. https://doi.org/10.1021/acs.energyfuels.0c04061
Guo, L., Tan, X., Mao, D., Zhao, T., Song, L., Kang, X., Wang, H., Sun, L., and Chu, W., Improved electrochemical activity of the Li2MnO3-like superstructure in high-nickel Li-rich layered oxide Li1.2Ni0.4Mn0.4O2 and its enhanced performances via tungsten doping, Electrochim. Acta, 2021, vol. 370, p. 137808. https://doi.org/10.1016/j.electacta.2021.137808
Bian, X., Zhang, R., and Yang, X., Effects of structure and magnetism on the electrochemistry of the layered Li1 + x(Ni0.5Mn0.5)1 – xO2 cathode material, Inorg. Chem., 2020, vol. 59, pp. 17535–17543. https://doi.org/10.1021/acs.inorgchem.0c02766
Shukla, A.K., Ramasse, Q.M., Ophus, C., Duncan, H., Hage. F., and Chen, G., Unravelling structural ambiguities in lithium- and manganese-rich transition metal oxides, Nat. Commun., 2015, vol. 6, p. 8711. https://doi.org/10.1038/ncomms9711
Genevois, C., Koga, H., Croguennec, L., Mentrier, M., Delmas, C., and Weill, F., Insight into the atomic structure of cycled lithium-rich layered oxide Li1.20Mn0.54Co0.13Ni0.13O2 using HAADF STEM and electron nanodiffraction, J. Phys. Chem. C, 2015, vol. 119, pp. 75–83. https://doi.org/10.1021/jp509388j
Li, J., Shunmugasundaram, R., Doig, R., and Dahn, J.R., In situ X-ray diffraction study of layered Li–Ni–Mn–Co oxides: effect of particle size and structural stability of core–shell materials, Chem. Mater., 2016, vol. 28, pp. 162–171. https://doi.org/10.1021/acs.chemmater.5b03500
Zheng, J., Gu, M., Genc, A., Xiao, J., Xu, P., Chen, X., Zhu, Z., Zhao, W., Pullan, L., Wang, C., and Zhang, J.-G., Mitigating voltage fade in cathode materials by improving the atomic level uniformity of elemental distribution, Nano Lett., 2014, vol. 14, pp. 2628–2635. https://doi.org/10.1021/nl500486y
Fu, F., Yao, Y., Wang, H., Xu, G.-L., Amine, K., Sun, S.-G., and Shao, M., Structure dependent electrochemical performance of Li-rich layered oxides in lithium-ion batteries, Nano Energy, 2017, vol. 35, pp. 370–378. https://doi.org/10.1016/j.nanoen.2017.04.005
Pechen, L.S., Makhonina, E.V., Rumyantsev, A.M., Koshtyal, Yu.M., Pervov, V.S., and Eremenko, I.L., Effect of the synthesis method on the functional properties of lithium-rich complex oxides Li1.2Mn0.54Ni0.13Co0.13O2, Russ. J. Inorg. Chem., 2018, vol. 63, no. 12, pp. 1534–1540. https://doi.org/10.1134/S0036023618120173
Chernyavsky, V., Kim, A., Koshtyal, Yu., Rumyantsev, A., Popovich, A., and Maximov, M.Yu., Structural features of complete and partial activation of Li-rich cathodes studied by in-situ XRD, Electrochim. Acta, 2022, vol. 414, p. 140237. https://doi.org/10.1016/j.electacta.2022.140237
Nachimuthu, S., Huang, H.-W., Lin, K.-Y., Yu, C., and Jiang, J.-C., Direct visualization of lattice oxygen evolution and related electronic properties of Li1.2Ni0.2Mn0.6O2 cathode materials, Appl. Surf. Sci., 2021, vol. 563, p. 150334. https://doi.org/10.1016/j.apsusc.2021.150334
Seo, D.-H., Lee, J., Urban, A., Malik, R., Kang, S., and Ceder, G., The structural and chemical origin of the oxygen redox activity in layered and cation-disordered Li-excess cathode materials, Nat. Chem., 2016, vol. 8, pp. 692–697. https://doi.org/10.1038/nchem.2524
Rana, J., Papp, J.K., Lebens-Higgins, Z., Zuba, M., Kaufman, L.A., Goel, A., Shmuch, R., Winter, M., Whittingham, M.S., Yang, W., McCloskey, B.D., and Piper, L.F.J., Quantifying the capacity contributions during activation of Li2MnO3, ACS Energy Lett., 2020, vol. 5, pp. 634–641. https://doi.org/10.1021/acsenergylett.9b02799
Akhilash, M., Salini, P.S., John, B., and Mercy, T.D., A journey through layered cathode materials for lithium ion cells – from lithium cobalt oxide to lithium-rich transition metal oxides, J. Alloys Compd., 2021, vol. 869, p. 159239. https://doi.org/10.1016/j.jallcom.2021.159239
Ye, D., Zeng, G., Nogita, K., Ozawa, K., Hankel, M., Searles, D., and Wang, L., Understanding the origin of Li2MnO3 activation in Li-rich cathode materials for lithium-ion batteries, Adv. Funct. Mater., 2015, vol. 25, pp. 7488–7496. https://doi.org/10.1002/adfm.201503276
Wang, T., Zhang, C., Li, S., Shen, X., Zhou, L., Huang, Q., Liang, C., Wang, Z., Wang, X., and Wei, W., Regulating anion redox and cation migration to enhance the structural stability of Li-rich layered oxides, ACS Appl. Mater. Interface, 2021, vol. 13, pp. 12159–12168. https://doi.org/10.1021/acsami.1c01351
Liang, X., Wu, H., Chen, H., and Mercy, T.D., Improving electrochemical performance of Li1.2Ni0.13Co0.13Mn0.54O2 cathode material by Al3+ doping, Int. J. Electrochem. Sci., 2016, vol. 11, pp. 9164–9174. https://doi.org/10.20964/2016.11.30
Kou, Y., Han, E., Zhu, L., Liu, L., and Zhang, Z., The effect of Ti doping on electrochemical properties of Li1.167Ni0.4Mn0.383Co0.05O2 for lithium-ion batteries, Solid State Ionics, 2016, vol. 296, pp. 154–157. https://doi.org/10.1016/j.ssi.2016.09.020
Pechen, L.S., Makhonina, E.V., Medvedeva, A.E., Rumyantsev, A.M., Koshtyal, Yu.M., Politov, Yu.A., Goloveshkin, A.S., and Eremenko, I.L., Effect of dopants on the functional properties of lithium-rich cathode materials for lithium-ion batteries, Russ. J. Inorg. Chem., 2021, vol. 66, no. 5, pp. 777–788. https://doi.org/10.1134/S0036023621050144
Makhonina, E., Pechen, L., Medvedeva, A., Politov, Yu., Rumyantsev, A., Koshtyal, Yu., Volkov, V., Goloveshkin, A., and Eremenko, I., Effects of Mg doping at different positions in Li-rich Mn-based cathode material on electrochemical performance, Nanomaterials, 2022, vol. 12, p. 156. https://doi.org/10.3390/nano12010156
Liu, Y., Zhang, Z., Gao, Y., Yang, G., Li, C., Zheng, J., Dou, A., Wang, Q., and Su, M., Mitigating the voltage decay and improving electrochemical properties of layered-spinel Li1.1Ni0.25Mn0.75O2.3 cathode material by Cr doping, J. Alloys Compd., 2016, vol. 657, pp. 37–43. https://doi.org/10.1016/j.jallcom.2015.10.060
Dong, S., Zhou, Y., Hai, C., Zeng, J., Sun, Y., Shen, Y., Li, X., Ren, X., Sun, G., Zhang, G., and Wu, Z., Understanding electrochemical performance improvement with Nb doping in lithium-rich manganese-based cathode materials, J. Power Sources, 2020, vol. 462, p. 228185. https://doi.org/10.1016/j.jpowsour.2020.228185
Huang, Z., Li, X., Liang, Y., He, Z., Chen, H., Wang, Z., and Guo, H., Structural and electrochemical characterization of Mg-doped Li1.2[Mn0.54Ni0.13Co0.13]O2 cathode material for lithium ion batteries, Solid State Ionics, 2015, vol. 282, pp. 88–94. https://doi.org/10.1016/j.ssi.2015.10.005
Jin, Y., Xu, Y., Ren, F., and Ren, P., Mg-doped Li1.133Ni0.2Co0.2Mn0.467O2 in Li site as high-performance cathode material for Li-ion batteries, Solid State Ionics, 2019, vol. 336, pp. 87–94. https://doi.org/10.1016/j.ssi.2019.03.020
Huang, Z., Wang, Z., Jing, Q., Guo, H., Li, X., and Yang, Z., Investigation on the effect of Na doping on structure and Li-ion kinetics of layered LiNi0.6Co0.2Mn0.2O2 cathode material, Electrochim. Acta, 2016, vol. 192, pp. 120–126. https://doi.org/10.1016/j.electacta.2016.01.139
Zhang, P., Zhai, X., Huang, H., Zhou, J., Li, X., He, X., and Guo, Z., Synergistic Na+ and F– co-doping modification strategy to improve the electrochemical performance of Li-rich Li1.20Mn0.54Ni0.13Co0.13O2 cathode, Ceram. Int., 2020, vol. 46, pp. 24723–24736. https://doi.org/10.1016/j.ceramint.2020.06.263
Taylor, Z.N., Perez, A.J., Coca-Clemente, J.A., Braga, F., Drewett, N.E., Pitcher, M.J., Thomas, W.J., Dyer, M.S., Collins, C., Zanella, M., Johnson, T., Tay, S., Tang, C., Dhanak, V.R., Claridge, J.B., Hardwick, L.J., and Rosseinsky, M.J., Stabilization of O–O bonds by d 0 cations in Li4 + xNi1 – xWO6 (0 ≤ x ≤ 0.25) rock salt oxides as the origin of large voltage hysteresis, J. Am. Chem. Soc., 2019, vol. 141, pp. 7333–7346. https://doi.org/10.1021/jacs.8b13633
Okubo, M. and Yamada, A., Molecular orbital principles of oxygen-redox battery electrodes, ACS Appl. Mater. Interfaces, 2017, vol. 9, pp. 36463–36472. https://doi.org/10.1021/acsami.7b09835
Liang, C., Kong, F., Longo, R.C., Zhang, C., Nie, Y., Zheng, Y., and Cho, K., Site-dependent multicomponent doping strategy for Ni-rich LiNi1 – 2yCoyMnyO2 (y = 1/12) cathode materials for Li-ion batteries, J. Mater. Chem. A, 2017, vol. 5, pp. 25303–25313.
Paulus, A., Hendrickx, M., Bercx, M., Karakulina, O.M., Kirsanova, M.A., Lamoen, D., Haderman, J., Abakumov, A.M., Van Bael, M.K., and Hardy, A., An in-depth study of Sn substitution in Li-rich/Mn-rich NMC as a cathode material for Li-ion batteries, Dalton Trans., 2020, vol. 49, pp. 10486–10497. https://doi.org/10.1039/D0DT01047B
Pechen, L.S., Makhonina, E.V., Rumyantsev, A.M., Koshtyal, Yu.M., Goloveshkin, A.S., Volkov, V.V., Politov, Yu.A., and Eremenko, I.L., Synthesis and electrochemical performance of Li-rich xLi2MnO3· (1 – x)LiMn1/3Ni1/3Co1/3O2 (x = 0.2–0.5) cathode materials for lithium-ion batteries, IOP Conf. Ser.: Mater. Sci. Eng., 2019, vol. 525, p. 012042. https://doi.org/10.1088/1757-899X/525/1/01204
ACKNOWLEDGMENTS
We are grateful to A.S. Goloveshkin (Nesmeyanov Institute of Organoelement Compounds, Russian Academy of Sciences) for performing phase analysis by X-ray diffraction.
This work was carried out using equipment at the Shared Physical Characterization Facilities Center, Kurnakov Institute of General and Inorganic Chemistry, Russian Academy of Sciences.
Funding
This work was supported by the Russian Science Foundation, project no. 20-13-00423.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Pechen, L.S., Makhonina, E.V., Medvedeva, A.E. et al. Influence of Tin and Titanium on the Electrochemical Performance of Lithium-Rich Cathode Materials. Inorg Mater 58, 1033–1042 (2022). https://doi.org/10.1134/S0020168522100119
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0020168522100119