Abstract—
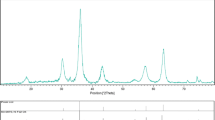
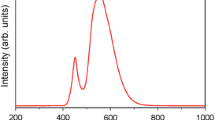
Nanoparticulate CoFe2O4 has been synthesized by the citrate combustion method. The nanopowder has been characterized from the viewpoint of its chemical homogeneity, particle size, dispersion, and morphological features. The results demonstrate that the CoFe2O4 nanopowder (with an average particle size on the order of 74 nm) is an effective catalyst for the oxidation of the organic pollutants methylene orange (degree of destruction of 76.6%) and 2,4-dinitrophenol (degree of destruction of 95.4%) in Fenton-like processes without additional heating or ultraviolet illumination.





Similar content being viewed by others
REFERENCES
Manova, E., Tsoncheva, T., Paneva, D., Mitov, I., Tenchev, K., and Petrov, L., Mechanochemically synthesized nano-dimensional iron–cobalt spinel oxides as catalysts for methanol decomposition, Appl. Catal., A, 2004, vol. 277, no. 1, pp. 119–127. https://doi.org/10.1016/j.apcata.2004.09.002
Kefeni, K.K., Msagati, A.M., and Mamba, B.B., Ferrite nanoparticles: synthesis, characterisation and applications in electronic device, Mater. Sci. Eng., B, 2017, vol. 215, pp. 37–55. https://doi.org/10.1016/j.mseb.2016.11.002
Petrova, E., Kotsikau, D., Pankov, V., and Fahmi, A., Influence of synthesis methods on structural and magnetic characteristics of Mg–Zn-ferrite nanopowders, J. Magn. Magn. Mater., 2019, vol. 473, pp. 85–91. https://doi.org/10.1016/j.jmmm.2018.09.128
Somnath, S., Indu, S., Kotnala, R.K., Singh, M., Kumar, A., Dhiman, P., Singh, V.P., Verma, K., and Kumar, G., Structural magnetic and Mössbauer studies of Nd-doped Mg–Mn ferrite nanoparticles, J. Magn. Magn. Mater., 2017, vol. 444, pp. 77–86. https://doi.org/10.1016/j.jmmm.2017.08.017
Rao, K.S., Nayakulu, S.V.R., Varma, M.C., Choudary, G.S.V.R.K., and Rao, K.H., Controlled phase evolution and the occurrence of single domain CoFe2O4 nanoparticles synthesized by PVA assisted sol–gel method, J. Magn. Magn. Mater., 2018, vol. 451, no. 1, pp. 602–608. https://doi.org/10.1016/j.jmmm.2017.11.069
Mittova, I.Ya., Perov, N.S., Tomina, E.V., Pan’kov, V.V., and Sladkopevtsev, B.V., Multiferroic nanocrystals and diluted magnetic semiconductors as a base for designing magnetic materials, Inorg. Mater., 2021, vol. 57, no. 13, pp. 22–48. https://doi.org/10.1134/S0020168521130033
Rehman, F., Sayed, M., Khan, J.A., Shah, L.A., Shah, N.S., Khan, H.M., and Khattak, R., Degradation of crystal violet dye by Fenton and photo-Fenton oxidation processes, Z. Phys. Chem., 2018, vol. 232, no. 12, pp. 1771–1786. https://doi.org/10.1515/zpch-2017-1099
Artemyanov, A.P., Zemskova, L.A., and Ivanov, V.V., Catalytic liquid-phase oxidation of phenol in water media using carbon fiber/(iron, iron oxide) catalyst, Izv. Vyssh. Uchebn. Zaved. Khim. Khim. Tekhnol., 2017, vol. 60, no. 8, pp. 88–95.
Rafferty, A., Prescott, T., and Brabazon, D., Sintering behaviour of cobalt ferrite ceramic, Ceram. Int., 2008, vol. 34, no. 1, pp. 15–21. https://doi.org/10.1016/j.ceramint.2006.07.012
Nabiyouni, G., Julaee, M., Ghanbari, D., Aliabadi, P.C., and Safaie, N., room temperature synthesis and magnetic property studies of Fe3O4 nanoparticles prepared by a simple precipitation method, J. Ind. Eng. Chem., 2015, vol. 21, pp. 599–603. https://doi.org/10.1016/j.jiec.2014.03.025
Ding, Z., Wang, W., Zhang, Y., Li, F., and Liu, J.P., Synthesis, characterization and adsorption capability for Congo red of CoFe2O4 ferrite nanoparticles, J. Alloys Compd., 2015, vol. 640, pp. 362–370. https://doi.org/10.1016/j.jallcom.2015.04.020
Larumbe, S., Perez-Landazabal, J.I., Pastor, J.M., and Gomez-Polo, C., Effect of a SiO2 coating on the magnetic properties of Fe3O4 nanoparticles, J. Appl. Phys., 2012, vol. 111, pp. 103911–103918. https://doi.org/10.1088/0953-8984/24/26/266007
Zakiyah, L.B., Saion, E., Al-Hada, N.M., Gharibshahi, E., Salem, A., Soltani, N., and Gene, S., Up-scalable synthesis of size-controlled copper ferrite nanocrystals by thermal treatment method, Mater. Sci. Semicond. Process., 2015, vol. 40, pp. 564–569. https://doi.org/10.1016/j.mssp.2015.07.027
Tian, Y., Yu, B., Li, X., Li, K., and Facile, J., Solvothermal synthesis of monodisperse Fe3O4 nanocrystals with precise size control of one nanometre as potential MRI contrast agents, Mater. Chem., 2011, vol. 21, pp. 2476–2481. https://doi.org/10.1039/C0JM02913K
Tomina, E.V., Perov, N.S., Mittova, I.Ya., Alekhina, Yu.A., Stekleneva, O.V., and Kurkin, N.A., Microwave synthesis and magnetic properties of bismuth ferrite nanopowder doped with cobalt, Russ. Chem. Bull., 2020, vol. 60, pp. 941–946. https://doi.org/10.1007/s11172-020-2852-1
Zhang, Z., Yao, G., Zhang, X., Ma, J., and Lin, H., Synthesis and characterization of nickel ferrite nanoparticles via planetary ball milling assisted solid-state reaction, Ceram Int., 015, vol. 41, pp. 4523–4530. https://doi.org/10.1016/j.ceramint.2014.11.147
Rashad, M.M., Soltan, S., Ramadan, A.A., Bekheet, M.F., and Rayan, D.A., Investigation of the structural, optical and magnetic properties of CuO/CoFe2O4 nanocomposites synthesized via simple microemulsion method, Ceram. Int., 2015, vol. 41, pp. 12237–12245. https://doi.org/10.1016/j.ceramint.2015.06.046
Shashank, D.B., Rakesh, K.S., Vivek, K., Nishant, K., and Shambhu, K., Tailoring the structural, optical and multiferroic properties of low temperature synthesized cobalt ferrite nanomaterials, by citrate precursor method, Mater. Today: Proc., 2021, vol. 46, no. 15, pp. 6527–6533. https://doi.org/10.1016/j.matpr.2021.04.001
Ali, T.M., Ismail, S.M., Mansour, S.F., Abdo, M.A., and Yehia, M., Physical properties of Al-doped cobalt nanoferrite prepared by citrate–nitrate auto combustion method, J. Mater. Sci.: Mater. Electron., 2021, vol. 32, pp. 3092–3103. https://doi.org/10.1007/s10854-020-05059-y
Mariosi, F.R., Venturini, J., Alexandre, C.V., and Bergmann, C.P., Lanthanum-doped spinel cobalt ferrite (CoFe2O4) nanoparticles for environmental applications, Ceram. Int., 2019, vol. 46, no. 3, pp. 2772–2779. https://doi.org/10.1016/j.ceramint.2019.09.266
Khodosova, N.A., Tomina, E.V., Bel’chinskaya, L.I., Zhabin, A.V., Kurkin, N.A., and Volkov, A.S., Physical and chemical characteristics of a nanocomposite sorbent, nontronite/CoFe2O4, Sorbtsionnye Khromatogr. Protsessy, 2021, vol. 21, no. 4, pp. 520–528. https://doi.org/10.17308/sorpchrom.2021.21/3636
JCPDC PCPDFWIN: A Windows Retrieval/Display Program for Accessing the ICDD PDF-2 Data Base, International Centre for Diffraction Data, 1997.
Brandon, D. and Kaplan, U., Microstructure of Materials. Research and Control Methods, West Sussex: Wiley, 1999, p. 384.
Roshanfekr, R.L., Farshi, G.B., Irani, M., Sadegh, S.M., and Haririan, I., Comparison study of phenol degradation using cobalt ferrite nanoparticles synthesized by hydrothermal and microwave methods, Desalination Water Treatment, 2014, vol. 56, no. 12, pp. 1–10. https://doi.org/10.1080/19443994.2014.97796
Papynov, E.K., Nomerovskii, A.D., Azon, A.S., Glavinskaya, V.O., Buravlev, I.Yu., Ognev, A.V., Samardak, A.S., Dran’kov, A.N., Krasitskaya, S.G., and Tananaev, I.G., Macroporous magnetic iron oxides and their composites for liquid-phase catalytic oxidation, Russ. J. Inorg. Chem., 2020, vol. 65, no. 11, pp. 1449–1460 https://doi.org/10.1134/S0036023620110157
Chomkitichai, W., Jansanthea, P., and Channei, D., Photocatalytic activity enhancement in methylene blue degradation by loading Ag nanoparticles onto α-Fe2O3, Russ. J. Inorg. Chem., 2021, vol. 66, pp. 1995–2003. https://doi.org/10.1134/S0036023621130027
ACKNOWLEDGMENTS
This study was carried out in part using equipment at the Shared Research Facilities Center, Voronezh State University (http://ckp.vsu.ru).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by O. Tsarev
Rights and permissions
About this article
Cite this article
Tomina, E.V., Kurkin, N.A. & Doroshenko, A.V. Synthesis of Nanoparticulate Cobalt Ferrite and Its Catalytic Properties for Fenton-Like Processes. Inorg Mater 58, 701–705 (2022). https://doi.org/10.1134/S0020168522070135
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0020168522070135