Abstract
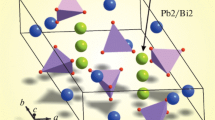
Pb10 – xSmx(GeO4)2 + x(VO4)4 – x (x = 0.2, 0.5, 0.7, 1.0) compounds with the apatite structure have been synthesized by firing appropriate mixtures of the PbO, Sm2O3, GeO2, and V2O5 oxides in air at temperatures from 773 to 1073 K. Their crystal structure has been studied using X-ray diffraction, and their high-temperature heat capacity (350–1000 K) has been determined by differential scanning calorimetry. The data have been used to evaluate their thermodynamic properties.




Similar content being viewed by others
REFERENCES
Kanazawa, T., Inorganic Phosphate Materials, Materials Science Monographs, vol. 52, Amsterdam: Elsevier, 1989.
Yano, T., Nabeta, Y., and Watanabe, A., A new crystal Pb5(GeO4)(VO4)2 for acousto-optic device applications, Appl. Phys. Lett., 1971, vol. 18, no. 12, pp. 570–571.
Gospodinov, M. and Sveshtarov, P., Growth of large Pb5(GeO4)(VO4)2 crystals, Cryst. Res. Technol., 1990, vol. 25, no. 3, pp. K58–K61.
Ignatov, A.V., Savinkova, G.M., Didorenko, E.G., et al., Isomorphous substitutions in the Pb(8 – x)GdxNa2(VO4)6O(x/2) system, Vestn. Donetsk. Nats. Univ., Ser. A: Estestv. Nauki, 2014, no. 1, pp. 152–156.
Kovács, L., Péter, Á., Gospodinov, M., and Capelleti, R., Hydroxyl ions in acousto-optic Pb5(GeO4)(VO4)2 and Bi2(MoO4)3 single crystals, Phys. Status Solidi, 2005, vol. 2, no. 1, pp. 689–692. https://doi.org/10.1002/pssc.200460267
Zhuravlev, V.D and Velikodny, Yu.A., Lead lanthanum and strontium lanthanum germanovanadates with apatite and oxyapatite structures, Russ. J. Inorg. Chem., 2009, vol. 54, no. 10, pp. 1551–1552. https://doi.org/10.1134/S0036023609100088
Savankova, T.M., Akselrud, L.G., Ardanova, L.I., et al., Synthesis, crystal structure refinement and electrical conductivity of Pb(8 – x)Na2Smx(VO4)6O(x/2), J. Chem., 2014, pp. 1–7. https://doi.org/10.1155/2014/263548
Chakroun-Ouadhour, E., Ternane, R., Ben Hassen-Chehimi, D., and Trabelsi-Ayadi, M., Synthesis, characterization and electrical properties of a lead sodium vanadate apatite, Mater. Res. Bull., 2008, vol. 43, pp. 2451–2456. https://doi.org/10.1016/j.materresbull.2007.07.030
Pasero, M., Kampf, A.R., Ferraris, C., et al., Nomenclature of the apatite super group minerals, Eur. J. Mineral., 2010, vol. 22, pp. 163–179. https://doi.org/10.1127/0935-1221/2010/0022-2022
Ptáćek, P., Opravil, T., Šoukal, F., et al., Formation of strontium–yttrium germanium anionic lacunar apatite (Sr2 + δY6.67 + (2δ/3)[GeO4]6O2δ) as the intermediate phase of oxygen-rich yttrium–germanium apatite (Y9.333 + ε[GeO4]6O2 + 3/2ε), Ceram. Int., 2017, vol. 43, pp. 7827–7838. https://doi.org/10.1016/j.ceramint.2017.03.097
Yablochkova, N.V., Synthesis of Pb8Pr2(GeO4)4(VO4)2 and refinement of its crystal structure, Russ. J. Inorg. Chem., 2013, vol. 58, no. 7, pp. 769–772. https://doi.org/10.1134/S0036023613070255
Ivanov, S.A., Crystal structure refinement of Pb5(GeO4)(VO4)2 using X-ray powder diffraction peak profiles, Zh. Strukt. Khim., 1990, vol. 31, no. 4, pp. 80–84.
Ivanov, S.A. and Zavodnik, V.E., Crystal structure of Pb5GeV2O12, Kristallografiya, 1989, vol. 34, no. 4, pp. 824–828.
Denisova, L.T., Kargin, Yu.F., Golubeva, E.O., et al., Heat capacity of Pb10 – xPrx(GeO4)2 + x(VO4)4 – x (x = 0, 1, 2, 3) apatites in the range 350–1050 K, Inorg. Mater., 2020, vol. 56, no. 10. pp. 1027–1032. https://doi.org/10.1134/S0020168520100039
Denisova, L.T., Golubeva, E.O., Belousova, N.V., et al., High-temperature heat capacity of Pb10 – xNdx-(GeO4)2 + x(VO4)4 – x (x = 0–3) apatites, Phys. Solid State, 2019, vol. 61, no. 7, pp. 1343–1346. https://doi.org/10.1134/S1063783419070060
Denisova, L.T., Kargin, Yu.F., Golubeva, E.O., et al., Heat capacity of Pb10 – xLax(GeO4)2 + x(VO4)4 – x (x = 0, 1, 2, 3) apatites in the range 320–1000 K, Inorg. Mater., 2019, vol. 55, no. 2, pp. 162–166. https://doi.org/10.1134/S002016851902002X
Bruker AXS TOPAS V4: General Profile and Structure Analysis Software for Powder Diffraction Data, Karlsruhe: Bruker, 2008.
Denisova, L.T., Irtyugo, L.A., Kargin, Yu.F., Beletskii, V.V., and Denisov, V.M., High-temperature heat capacity and thermodynamic properties of Tb2Sn2O7, Inorg. Mater., 2017, vol. 53, no. 1, pp. 93–95. https://doi.org/10.1134/S0020168517010046
Savankova, T.M., Ignatov, A.V., Utochkin, D.M., and Get’man, E.I., Synthesis and characterization of Pb(8 – x)EuxNa2(VO4)6O(x/2) solid solutions, Nauk. Pratsi Khim. Tekhnol., 2014, no. 2, pp. 78–82.
Koumiri, M.E., Oishi, S., Sato, S., et al., The crystal structure of lacunar apatite NaPb4(PO4)3, Mater. Res. Bull., 2000, vol. 35, pp. 503–513.
Shannon, R.D., Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides, Acta Crystallogr., Sect. A: Cryst. Phys., Diffr., Theor. Gen. Crystallogr., 1976, vol. 32, no. 5, pp. 751–767.
Chudnenko, K.V., Termodinamicheskoe modelirovanie v geokhimii: teoriya, algoritmy, programmnoe obespechenie, prilozheniya (Thermodynamic Modeling in Geochemistry: Theory, Algorithms, Software, and Applications), Novosibirsk: Geo, 2010.
Maier, C.G. and Kelley, K.K., An equation for the representation of high temperature heat content data, J. Am. Chem. Soc., 1932, vol. 54, no. 8, pp. 3243–3246. https://doi.org/10.1021/ja01347a029
Denisova, L.T., Molokeev, M.S., Aleksandrovskii, A.S., et al., Crystal structure, luminescence, and thermodynamic properties of Pb10 – xEux(GeO4)2 + x(VO4)4 – x (x = 0.1, 0.2, 0.3) substituted apatites, Inorg. Mater., 2021, vol. 57, no. 11, pp. 1158–1166. https://doi.org/10.1134/S0020168521110030
ACKNOWLEDGMENTS
We are grateful to the Krasnoyarsk Regional Shared Research Facilities Center, Krasnoyarsk Scientific Center (Federal Research Center), Siberian Branch, Russian Academy of Sciences.
Funding
This work was supported in part by the Russian Federation Ministry of Science and Higher Education as part of the state research target for the Siberian Federal University federal state autonomous educational institution of higher education, project no. FSRZ-2020-0013.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by O. Tsarev
Rights and permissions
About this article
Cite this article
Denisova, L.T., Molokeev, M.S., Kargin, Y.F. et al. Synthesis, Crystal Structure, and High-Temperature Heat Capacity of Pb10 – xSmx(GeO4)2 + x(VO4)4 – x (x = 0.2, 0.5, 0.7, 1.0) Apatites from 350 to 1000 K. Inorg Mater 58, 831–837 (2022). https://doi.org/10.1134/S0020168522070081
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0020168522070081