Abstract—
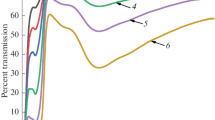
Optical transmission of Cu2+-doped glass with the composition (TeO2)0.72(ZnO)0.18(MoO3)0.10 has been studied at dopant concentrations from 0.008 to 0.250 wt % and wavelengths from 0.45 to 2.80 μm. The transmission spectra of the glasses each have one strong absorption band peaking at ~819 nm. From the composition dependence of the absorption coefficient for a series of Cu2+-doped glass samples, we have calculated the specific absorption coefficient of Cu2+ ions, 4070 ± 83 dB/(km ppm) at 819 nm, and obtained its spectral dependence in the wavelength range studied.




Similar content being viewed by others
REFERENCES
Rivera, V.A.G. and Manzani, D., Technological Advances in Tellurite Glasses: Properties, Processing, and Applications, Cham: Springer, 2017, p. 344.
Khatir, S., Bolka, J., Capoen, B., Turrell, S., and Bouazaoui, M., Raman spectroscopic characterization of Er3+-doped tellurite-based glasses, J. Mol. Struct., 2001, vols. 563–564, no. 5, pp. 283–287. https://doi.org/10.1016/S0022-2860(01)00440-9
Strutynski, C., Desevedavy, F., Lemière, A., Jules, J.-C., Gadret, G., Cardinal, T., Smektala, F., and Danto, S., Tellurite-based core–clad dual-electrodes composite fibers, Opt. Mater. Express, 2017, vol. 7, no. 5, pp. 1503–1508. https://doi.org/10.1364/OME.7.001503
Himamaheswara Rao, V., Syam Prasad, P., Mohan Babu, M., Venkateswara Rao, P., Satyanarayana, T., Santos, L.F., and Veeraiah, N., Spectroscopic studies of Dy3+ ion doped tellurite glasses for solid state lasers and white LEDs, Spectrochim. Acta, Part A, 2018, vol. 188, pp. 516–524. https://doi.org/10.1016/j.saa.2017.07.013
El-Mallawany, R.A.H., Tellurite Glass Smart Materials: Applications in Optics and Beyond, Cham: Springer, 2018.
El-Mallawany, R.A.H., Tellurite Glasses Handbook: Physical Properties and Data, Boca Raton: CRC, 2011, p. 532.
Zhang, W. and Halasyamani, P.S., Top-seeded solution crystal growth of noncentrosymmetric and polar Zn2TeMoO7 (ZTM), J. Solid State Chem., 2015, vol. 236, pp. 32–38. https://doi.org/10.1016/j.jssc.2015.08.044
Liu, J.L., Wang, W.C., Xiao, Y.B., Huang, S.J., Mao, L.Y., and Zhang, Q.Y., Nd3+-doped TeO2–MoO3–ZnO tellurite glass for a diode-pump 1.06 μm laser, J. Non-Cryst. Solids, 2019, vol. 506, pp. 32–38. https://doi.org/10.1016/j.jnoncrysol.2018.11.030
Snopatin, G.E., Plotnichenko, V.G., Volkov, S.A., Dorofeev, V.V., Dianov, E.M., and Churbanov, M.F., Extinction coefficient of Ni2+ in (TeO2)0.78(WO3)0.2 glass, Inorg. Mater., 2010, vol. 46, no. 8, pp. 914–917. https://doi.org/10.1134/S0020168510080212
Zamyatin, O.A., Churbanov, M.F., Plotnichenko, V.G., Sibirkin, A.A., and Goreva, I.G., Specific absorption coefficient of nickel in (TeO2)0.80(MoO3)0.20 glass, Inorg. Mater., 2015, vol. 51, no. 3, pp. 278–282. https://doi.org/10.1134/S0020168515030188
Zamyatin, O.A., Churbanov, M.F., Plotnichenko, V.G., Kharakhordin, A.V., Sibirkin, A.A., and Fedotova, I.G., Specific absorption coefficient of cobalt(II) in (TeO2)0.80(MoO3)0.20 glass, Inorg. Mater., 2015, vol. 51, no. 6, pp. 631–634. https://doi.org/10.1134/S0020168515060199
Zamyatin, O.A., Churbanov, M.F., Plotnichenko, V.G., Sibirkin, A.A., Fedotova, I.G., and Gavrin, S.A., Specific absorption coefficient of copper in (TeO2)0.80(MoO3)0.20 glass, Inorg. Mater., 2015, vol. 51, no. 12, pp. 1283–1287. https://doi.org/10.1134/S0020168515110163
Dorofeev, V.V., Moiseev, A.N., Churbanov, M.F., Snopatin, G.E., Chilyasov, A.V., Kraev, I.A., Lobanov, A.S., Kotereva, T.V., Ketkova, L.A., Pushkin, A.A., Gerasimenko, V.V., Plotnichenko, V.G., Kosolapov, A.F., and Dianov, E.M., High-purity TeO2–WO3–(La2O3,Bi2O3) glasses for fiber-optics, Opt. Mater., 2011, vol. 33, no. 12, pp. 1911–1915. https://doi.org/10.1016/j.optmat.2011.03.032
Moiseev, A.N., Dorofeev, V.V., Chilyasov, A.V., Kraev, I.A., Churbanov, M.F., Kotereva, T.V., Pimenov, V.G., Snopatin, G.E., Pushkin, A.A., Gerasimenko, V.V., Kosolapov, A.F., Plotnichenko, V.G., and Dianov, E.M., Production and properties of high purity TeO2–ZnO–Na2O–Bi2O3 and TeO2–WO3–La2O3–MoO3 glasses, Opt. Mater., 2011, vol. 33, no. 12, pp. 1858–1861. https://doi.org/10.1016/j.optmat.2011.02.042
Lyubchanskii, I.L., Dadoenkova, N.N., Lyubchanskii, M.I., Shapovalov, E.A., and Rasing, T., Magnetic photonic crystals, J. Phys. D: Appl. Phys., 2003, vol. 36, no. 18, pp. R277–R287. https://doi.org/10.1088/0022-3727/36/18/R01
Kozak, A.J., Wieczorek-Ciurowa, K., and Kozak, A., The thermal transformations in Zn(NO3)2⋅H2O (1 : 6) system, J. Therm. Anal. Calorim., 2003, vol. 74, no. 2, pp. 497–502. https://doi.org/10.1023/B:JTAN.0000005186.15474.be
Małecki, A., Gajerski, R., Łabuś, S., Prochowska-Klisch, B., and Wojciechowski, K.T., Mechanism of thermal decomposition of d-metals nitrates hydrates, J. Therm. Anal. Calorim., 2000, vol. 60, no. 1, pp. 17–23. https://doi.org/10.1023/A:1010155931266
Živković Ž.D., Živković, D.T., and Grujičić, D.B., Kinetics and mechanism of the thermal decomposition of M(NO3)2⋅nH2O (M = Cu, Co, Ni), J. Therm. Anal. Calorim., 1998, vol. 53, no. 2, pp. 617–623. https://doi.org/10.1023/A:1010170231923
Nikolic, R., Zec, S., Maksimovic, V., and Mentus, S., Physico-chemical characterization of thermal decomposition course in zinc nitrate–copper nitrate hexahydrates, J. Therm. Anal. Calorim., 2006, vol. 86, no. 2, pp. 423–428. https://doi.org/10.1007/s10973-005-7237-z
Ahmed, M.A.K., Fjellvåg, H., and Kjekshus, A., Synthesis, structure and thermal stability of tellurium oxides and oxide sulfate formed from reactions in refluxing sulfuric acid, J. Chem. Soc., Dalton Trans., 2000, no. 24, pp. 4542–4549. https://doi.org/10.1039/B005688J
Von Rosicky, J., Loub, J., and Pavel, J., Ber die thermische Zersetzung der Orthotellursäure und die Verbindung Te2O5, Z. Anorg. Allg. Chem., 1965, vol. 334, nos. 5–6, pp. 312–320. https://doi.org/10.1002/zaac.19653340512
Bart, J.C.J., Bossi, A., Perissinoto, P., Castellan, A., and Giordano, N., Some observations on the thermochemistry of telluric acid, J. Therm. Anal., 1975, vol. 8, no. 2, pp. 313–327. https://doi.org/10.1007/BF01904009
Bayer, G., On the polymorphism of orthotelluric acid, H6TeO6, J. Less-Common. Met., 1968, vol. 16, no. 3, pp. 215–222. https://doi.org/10.1016/0022-5088(68)90017-9
Feger, C.R., Schimek, G.L., and Kolis, J.W., Hydrothermal synthesis and characterization of M2Te3O8 (M = Mn, Co, Ni, Cu, Zn): a series of compounds with the spiroffite structure, J. Solid State Chem., 1999, vol. 143, no. 2, pp. 246–253. https://doi.org/10.1006/jssc.1998.8101
Pertlik, F., Dimorphism of hydrothermal synthesized copper tellurite, CuTeO3: the structure of a monoclinic representative, J. Solid State Chem., 1987, vol. 71, no. 2, pp. 291–295. https://doi.org/10.1016/0022-4596(87)90236-2
Stavrakeva, D., Ivanova, Y., and Pyrov, Y., New data on the composition of the crystalline phases in the Cu–Te–O system, J. Mater. Sci., 1990, vol. 25, no. 4, pp. 2175–2180. https://doi.org/10.1007/BF01045785
Zhu, X., Wang, Z., Su, X., and Vilarinho, P.M., New Cu3TeO6 ceramics: phase formation and dielectric properties, ACS Appl. Mater. Interfaces, 2014, vol. 6, no. 14, pp. 11326–11332. https://doi.org/10.1021/am501742z
Yoshida, T., Hirashima, H., and Kato, M., Electrical conductivity of glass and crystallized glass of system CuO−V2O5−TeO2, J. Ceram. Association, 1985, vol. 93, no. 1077, pp. 244–251. https://doi.org/10.2109/jcersj1950.93.1077_244
Gayathri Pavani, P., Vijaya Kumar, R., and Chandra Mouli, V., Characterization of ZnO based boro tellurite glass system, Phys. Chem. Glasses: Eur. J. Glass Sci. Technol., 2016, vol. 57, no. 2, pp. 104–110. https://doi.org/10.13036/17533562.57.2.013
Upender, G., Devi, C.S., Kamalaker, V., and Mouli, V.C., The structural and spectroscopic investigations of ternary tellurite glasses, doped with copper, J. Alloys Compd., 2011, vol. 509, no. 19, pp. 5887–5892. https://doi.org/10.1016/j.jallcom.2011.03.001
Sreedhar, B., Rao, J.L., and Lakshman, S.V.J., Electron spin resonance and optical absorption spectra of Cu2+ ions in alkali zinc borosulphate glasses, J. Non-Cryst. Solids, 1990, vol. 124, nos. 2–3, pp. 216–220. https://doi.org/10.1016/0022-3093(90)90265-N
Narendra, G.L., Sreedhar, B., Rao, J.L., and Lakshman, S.V.J., Electron spin resonance and optical absorption spectra of Cu2+ ions in Na2SO4−ZnSO4 glasses, J. Mater. Sci., 1991, vol. 26, no. 19, pp. 5342–5346. https://doi.org/10.1007/BF01143231
Ramadevudu, G., Shareefuddin, M., Sunitha Bai, N., Lakshmipathi Rao, M., and Narasimha Chary, M., Electron paramagnetic resonance and optical absorption studies of Cu2+ spin probe in MgO–Na2O–B2O3 ternary glasses, J. Non-Cryst. Solids, 2000, vol. 278, nos. 1–3, pp. 205–212. https://doi.org/10.1016/S0022-3093(00)00255-6
Rayan, D.A., Elbashar, Y.H., Rashad, M.M., and El-Korashy, A., Optical spectroscopic analysis of cupric oxide doped barium phosphate glass for bandpass absorption filter, J. Non-Cryst. Solids, 2013, vol. 382, pp. 52–56. https://doi.org/10.1016/j.jnoncrysol.2013.10.002
Stefan, R., Culea, E., and Pascuta, P., The effect of copper ions addition on structural and optical properties of zinc borate glasses, J. Non-Cryst. Solids, 2012, vol. 358, no. 4, pp. 839–846. https://doi.org/10.1016/j.jnoncrysol.2011.12.079
Upender, G., Prasad, M., and Mouli, V.C., Vibrational, EPR and optical spectroscopy of the Cu2+ doped glasses with (90 − x)TeO2−10GeO2−xWO3 (7.5 ≤ x ≤ 30) composition, J. Non-Cryst. Solids, 2011, vol. 357, no. 3, pp. 903–909. https://doi.org/10.1016/j.jnoncrysol.2010.12.001
Kamalaker, V., Upender, G., Prasad, M., and Mouli, V.C., Infrared, ESR and optical absorption studies of Cu2+ ions doped in TeO2−ZnO−NaF glass system, Indian J. Pure Appl. Phys., 2010, vol. 48, no. 10, pp. 709–715.
Schultz, P.C., Optical absorption of the transition elements in vitreous silica, J. Am. Ceram. Soc., 1974, vol. 57, no. 7, pp. 309–313. https://doi.org/10.1111/j.1151-2916.1974.tb10908.x
Newns, G.R., Pantelis, P., Wilson, J.L., Uffen, R.W.J., and Worthington, R., Absorption losses in glasses and glass fibre waveguides, Opto-electronics, 1973, vol. 5, no. 4, pp. 289–296. https://doi.org/10.1007/BF02057128
Spierings, G.A.C.M., Optical absorption of transition metals in alkali lime germanosilicate glasses, J. Mater. Sci., 1979, vol. 14, no. 10, pp. 2519–2521. https://doi.org/10.1007/BF00737045
Keppler, H., Crystal field spectra and geochemistry of transition metal ions in silicate melts and glasses, Am. Mineral., 1992, vol. 77, nos. 1–2, pp. 62–75.
France, P.W., Carter, S.W., and Williams, J.R., Effects of atmosphere control on the oxidation states of 3d transition metals in ZrF4 based glasses, Mater. Sci. Forum, 1985, vols. 5–6, pp. 353–359. https://doi.org/10.4028/www.scientific.net/MSF.5-6.353
Zamyatin, O.A., Plotnichenko, V.G., Churbanov, M.F., Zamyatina, E.V., and Karzanov, V.V., Optical properties of zinc tellurite glasses doped with Cu2+ ions, J. Non-Cryst. Solids, 2018, vol. 480, pp. 81–89. https://doi.org/10.1016/j.jnoncrysol.2017.08.025
Funding
This work was supported by the Russian Federation Ministry of Science and Higher Education, state research target, basic research, project no. 0729-2020-0039.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by O. Tsarev
Rights and permissions
About this article
Cite this article
Zamyatin, O.A., Leksakov, D.A. & Nosov, Z.K. Extrinsic Absorption by Copper(II) Ions in Molybdenum-Containing Zinc Tellurite Glass. Inorg Mater 57, 1178–1183 (2021). https://doi.org/10.1134/S0020168521110145
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0020168521110145