Abstract—
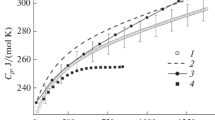
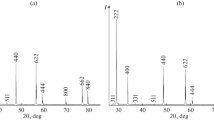
The molar heat capacity and lattice parameter of samarium hafnate with the pyrochlore structure have been measured as functions of temperature in the ranges 320–1300 and 298–1273 K, and its thermal expansion coefficients have been evaluated.



Similar content being viewed by others
REFERENCES
Isupova, E.N., Glushkova, V.B., and Keler, K.E., The HfO2–Sm2O3 system in solid phases in the HfO2-rich region, Izv. Akad. Nauk SSSR. Neorg. Mater., 1968, vol. 4, pp. 1330–1331.
Duran, P., The system hafnia–samaria, J. Am. Ceram. Soc., 1979, vol. 62, pp. 9–12.https://doi.org/10.1111/j.1151-2916.1979.tb18794.x
Shevchenko, A.V., Lopato, L.M., and Nazarenko, L.V., The systems of HfO2 with oxides of samarium, gadolinium, terbium and dysprosium at high temperatures, Izv. Akad. Nauk SSSR, Neorg. Mater., 1984, vol. 20, pp. 1862–1866.
Paputsky, Yu.N., Krzizanovskaya, V.A., and Glushkova, V.B., Formation enthalpy for rare earth hafnates and zirconates, Izv. Akad. Nauk SSSR, Neorg. Mater., 1974, vol. 10, pp. 1551–1552.
Arsen’ev, P.A., Glushkova, V.B., Evdokimov, A.A., et al., Soedineniya redkozemel’nykh elementov. Tsirkonaty, gafnaty, niobaty, tantalaty, antimonaty (Rare-Earth Compounds: Zirconates, Hafnates, Niobates, Tantalates, and Antimonates), Moscow: Nauka, 1985.
Andrievskaya, E.R., Phase equilibria in the refractory oxide systems of zirconia, hafnia and yttria with rare-earth oxides, J. Eur. Ceram. Soc., 2008, vol. 28, pp. 2363–2388.https://doi.org/10.1016/jeurceramsoc.2008.01.009
Stanec, C.R. and Grimes, R.W., Prediction of rare-earth A2Hf2O7 pyrochlore phases, J. Am. Ceram. Soc., 2002, vol. 85, pp. 2139–2141.https://doi.org/10.1111/j.1151-2916.2002.tb00423.x
Rushton, M.J.D., Grimes, R.W., Stanek, C.R., and Owens, S., Predicted pyrochlore to fluorite disorder temperature for A2Zr2O7 compositions, J. Mater. Res., 2004, vol. 19, pp. 1603–1604.https://doi.org/10.1557/jmr.2004.0231
Subramanian, M.A., Aravamudan, G., and Subba Rao, G.V., Oxide pyrochlores—a review, Prog. Solid State Chem., 1983, vol. 15, pp. 55–143.https://doi.org/10.1016/0079-6786(83)90001-8
Jiang, C., Stanek, C.R., Sickafus, K.E., and Uberiaga, B.P., First-principles prediction of disordering tendencies in pyrochlore oxides, Phys. Rev. B: Condens. Matter Mater. Phys., 2009, vol. 79, paper 104203.https://doi.org/10.1103/PhysRevB.79.104203
Popov, V.V., Menushenkov, A.P., Yaroslavtsev, A.A., et al., Fluorite–pyrochlore phase transition in nanostructured Ln2Hf2O7 (Ln = La–Lu), J. Alloys Compd., 2016, vol. 689, pp. 669–679.https://doi.org/10.1016/j.jallcom.2016.08.019
Guskov, V.N., Gavrichev, K.S., Gagarin, P.G., and Guskov, A.V., Thermodynamic functions of complex zirconia based lanthanide oxides—pyrochlores Ln2Zr2O7 (Ln = La, Pr, Sm, Eu, Gd) and fluorites Ln2O3 · 2ZrO2 (Ln = Tb, Ho, Er, Tm), Russ. J. Inorg. Chem., 2019, vol. 64, pp. 1265–1281.https://doi.org/10.1134/S0036023619100048
Vaßen, R., Jarligo, M.O., Steinke, T., et al., Overview on advanced thermal barrier coatings, Surf. Coat. Technol., 2010, vol. 205, pp. 938–942.https://doi.org/10.1016/j.surfcoat.2010.08.151
Clarke, D.R. and Phillpot, S.R., Thermal barrier coating materials, Mater. Today, 2005, vol. 8, pp. 22–29.https://doi.org/10.1016/s1369-7021(05)70934-2
Poerschke, D.L., Jackson, R.W., and Levi, C.G., Silicate deposit degradation of engineered coatings in gas turbines: progress toward models and materials solutions, Ann. Rev. Mater. Res., 2017, vol. 47, pp. 297–330.https://doi.org/10.1146/annurev-matsci-010917-105000
Yamamura, H., Electrical conductivity anomaly around fluorite–pyrochlore phase boundary, Solid State Ionics, 2003, vol. 158, pp. 359–365.https://doi.org/10.1016/s0167-2738(02)00874-3
Shlyakhtina, A.V. and Shcherbakova, L.G., Polymorphism and high-temperature conductivity of Ln2M2O7 (Ln = Sm–Lu; M = Ti, Zr, Hf) pyrochlores, Solid State Ionics, 2011, vol. 192, pp. 200–204. https://doi.org/1016/j.ssi.2010.07.013
Risovany, V.D., Zakharov, A.V., Muraleva, E.M., et al., Dysprosium hafnate as absorbing material for control rods, J. Nucl. Mater., 2006, vol. 355, pp. 163–170.https://doi.org/10.1016/j.jnucmat.2006.05.029
Ewing, R.C., Weber, W.J., and Lian, J., Nuclear waste disposal-pyrochlore (A2B2O7): nuclear waste form for the immobilization of plutonium and “minor” actinides, J. Appl. Phys., 2004, vol. 95, pp. 5949–5971.https://doi.org/10.1063/1.1707213
Kandan, R., Prabhakara Reddy, B., Panneerselvam, G., and Mudali, U.K., Enthalpy measurements on rare earth hafnates RE2O3⋅2HfO2 (s) (RE = Sm, Eu, Dy), J. Therm. Anal. Calorim., 2017, vol. 131, pp. 2687–2692.https://doi.org/10.1007/s10973-017-6802-6
Lópes-Cota, F.A., Cepeda-Sánchez, N.M., Díaz-Guillén, J.A., et al., Electrical and thermophysical properties of mechanochemically obtained lanthanide hafnates, J. Am. Ceram. Soc., 2017, vol. 100, pp. 1994–2004.https://doi.org/10.1111/jace.14712
Guskov, V.N., Tyurin, A.V., Guskov, A.V., et al., Thermal expansion and thermodynamic properties of gadolinium hafnate ceramics, Ceram. Int., 2020, vol. 46, pp. 12822–12827.https://doi.org/10.1016/j.ceramint.2020.02.052
Kutty, K.V.G., Rajagopalan, S., Mathews, C.K., and Varadaraju, U.V., Thermal expansion behaviour of some rare earth oxide pyrochlore, Mater. Res. Bull., 1994, vol. 29, pp. 759–766.https://doi.org/10.1016/0025-5408(94)90201-1
Mikuśkiewicz, M., Migas, D., and Moskal, G., Synthesis and thermal properties of zirconate, hafnate and cerate of samarium, Surf. Coat. Technol., 2018, vol. 354, pp. 66–75.https://doi.org/10.1016/j.surfcoat.2018.08.096
Gagarin, P.G., Tyurin, A.V., Guskov, V.N., et al., Thermodynamic properties and thermal expansion of Tm2O3 · 2ZrO2 solid solution, Russ. J. Inorg. Chem., 2018, vol. 63, pp. 1478–1483.https://doi.org/10.1134/S0036023618110050
Wieser, M.E., Atomic weights of the elements 2005 (IUPAC technical report), Pure Appl. Chem., 2006, vol. 78, pp. 2051–2066.https://doi.org/10.1351/pac200678112051
Ryumin, M.A., Nikiforova, G.E., Tyurin, A.V., Khoroshilov, A.V., Kondrat’eva, O.N., Guskov, V.N., and Gavrichev, K.S., Heat capacity and thermodynamic functions of La2Sn2O7, Inorg. Mater., 2020, vol. 56, no. 1, pp. 97–104.https://doi.org/10.1134/S00201685200101148
Kolomiets, T.Yu., Tel’nova, G.B., Ashmarin, A.A., Chelpanov, V.I., and Solntsev, K.A., Synthesis and sintering of submicron Nd:YAG particles prepared from carbonate precursors, Inorg. Mater., 2017, vol. 53, no. 8, pp. 874–882. https://doi.org/10.1134/S0020168517080076
Gagarin, P.G., Guskov, A.V., Guskov, V.N., et al., Dysprosium orthotantalate ceramics: thermal expansion and heat capacity, Ceram. Int., 2021, vol. 47, pp. 2892–2896.https://doi.org/10.1016/j.ceramint.2020.09072
Shlyakhtina, A.V., Knotko, A.V., Boguslavskii, M.V., et al., Effect of non-stoichiometry and synthesis temperature on the structure and conductivity of Ln2 + xM2 − xO7 − x/2 (Ln = Sm–Gd; M = Zr, Hf; x = 0–0.286), Solid State Ionics, 2007, vol. 17, pp. 59–66.https://doi.org/10.1016/j.ssi.2006.11.001
Maier, C.G. and Kelley, K.K., An equation for representation of high temperature heat content data, J. Am. Chem. Soc., 1932, vol. 54, pp. 3243–3246.https://doi.org/10.1021/ja01347a029
ACKNOWLEDGMENTS
This work was carried out using equipment of the JRC PMR IGIC RAS. The assistance of PhD A.A. Ashmarin in HTXRD studies is kindly appreciated.
Funding
This work was supported by the Russian Science Foundation, grant no. 18-13-00025.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Guskov, A.V., Gagarin, P.G., Guskov, V.N. et al. Heat Capacity and Thermal Expansion of Samarium Hafnate. Inorg Mater 57, 1015–1019 (2021). https://doi.org/10.1134/S0020168521100046
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0020168521100046