Abstract
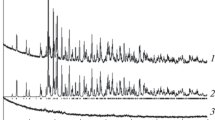
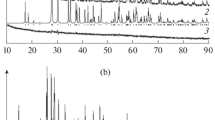
The europium gallium germanate EuGaGe2O7 has been prepared by solid-state reaction in air in the temperature range 1273–1473 K using a stoichiometric mixture of Eu2O3, Ga2O3, and GeO2. Its crystal structure has been determined by X-ray diffraction (sp. gr. P21/c, a = 7.1693(7) Å, b = 6.57008(6) Å, c = 12.7699(1) Å, β = 117.4522(5)°, V = 533.768(8) Å3). The heat capacity of polycrystalline samples has been determined by differential scanning calorimetry in the temperature range 350–1053 K and the experimental data have been used to calculate the thermodynamic properties (enthalpy increment, entropy change, and reduced Gibbs energy change) of EuGaGe2O7.



Similar content being viewed by others
REFERENCES
Jarchow, O., Klaska, K.-H., and Schenk, H., REAlGe2O7 – new compounds of rare earth germinates, Naturwissenschaften, 1981, vol. 68, pp. 475–476.
Kaminskii, A.A., Mill, B.V., Butashin, A.V., et al., Germanates with NdAlGe2O7-type structure. Synthesis, crystal structure, absorption–luminescence properties, and stimulated emission of their activator, Nd3+ ions, Phys. Status Solidi A, 1987, vol. 103, pp. 575–592.
Cascales, C., Puebla, G., Klimin, S., et al., Magnetic ordering in the rare earth iron germanates HoFeGe2O7 and ErFeGe2O7, Chem. Mater., 1999, vol. 11, pp. 2520–2526.
Lozano, G., Cascales, C., Zaldo, C., and Porcher, P., Measurement and simulation of the energy levels of R = Pr3+ and Nd3+ in GaRGe2O7, J. Alloys Compd., 2000, vols. 303–304, pp. 349–354.
Kaminakii, A.A., Rhee, H., Lux, O., et al., Monoclinic LaGaGe2O7:Nd3+ – a novel SRS- and SE-active crystal with high-order Stokes and anti-Stokes picosecond χ(3)-nonlinear lasing, Laser Phys. Lett., 2013, vol. 10, paper 075 803.https://doi.org/10.1088/1612-2011/10/7/075803
Jarchow, O., Klaska, K.-H., and Schenk-Strauß, H., Die Kristallstrukturen von NdAlGe2O7 und NdGaGe2O7, Z. Kristallogr., 1985, vol. 172, pp. 159–166.
Cascales, C., Fernȧndez-Diaz, M.T., Monge, M.A., and Bucio, L., Crystal structure and low-temperature magnetic ordering in rare earth iron germanates RFeGe2O7, R = Y, Pr, Dy, Tm, and Yb, Chem. Mater., 2002, vol. 14, pp. 1995–2003.https://doi.org/10.1021/cm0111332
Buciot, L., Cascales, C., Alonso, J.A., and Rasines, I., Neutron diffraction refinement characterization of FeRGe2O7 (R = La, Pr, Nd, Gd), J. Phys.: Condens. Matter, 1996, vol. 8, pp. 2641–2653.
Juarez-Arellano, E.A., Campa-Molina, J., Ulloa-Godinez, S., et al., Crystallochemistry of thortveitite-like and thortveitite-type compounds, Mater. Res. Soc. Symp., 2005, vol. 848, pp. FF6.15.1–FF6.15.8.
Denisova, L.T., Kargin, Yu.F., Irtyugo, L.A., et al., Heat capacity of In2Ge2O7 and YInGe2O7 from 320 to 1000 K, Inorg. Mater., 2018, vol. 54, no. 12, pp. 1245–1249.https://doi.org/10.1134/S0020168518120026
Denisova, L.T., Irtyugo, L.A., Belousova, N.V., et al., High temperature heat capacity and thermodynamic properties of Tm2Ge2O7 and TmInGe2O7 in the region of 350–1000 K, Russ. J. Phys. Chem. A, 2019, vol. 93, no. 3, pp. 598–601.https://doi.org/10.1134/S003602441903004X
Becker, U.W. and Felsche, J., Phases and structural relations of the earth germanates RE2Ge2O7, RE = La–Lu, J. Less-Common Met., 1987, vol. 128, pp. 269–280.
Denisova, L.T., Irtyugo, L.A., Kargin, Yu.F., et al., High-temperature heat capacity and thermodynamic properties of Tb2Sn2O7, Inorg. Mater., 2017, vol. 53, no. 1, pp. 93–95.https://doi.org/10.1134/S0020168517010046
Bruker AXS TOPAS V4: General Profile and Structure Analysis Software for Powder Diffraction Data. User’s Manual, Karlsruhe: Bruker AXS, 2008.
Maier, C.G. and Kelley, K.K., An equation for the representation of high temperature heat content data, J. Am. Chem. Soc., 1932, vol. 54, no. 8, pp. 3243–3246.
Denisova, L.T., Irtyugo, L.A., Kargin, Yu.F., et al., Synthesis and High-Temperature Heat Capacity of Sm2Ge2O7 and Eu2Ge2O7, Inorg. Mater., 2018, vol. 54, no. 2, pp. 167–170.https://doi.org/10.1134/S0020168518020048
Leitner, J., Chuchvalec, P., Sedmidubský, D., et al., Estimation of heat capacities of solid mixed oxides, Thermochim. Acta, 2003, vol. 395, nos. 1–2, pp. 27–46.
Kumok, V.N., Problem of matching techniques for evaluating thermodynamic characteristics, in Pryamye i obratnye zadachi khimicheskoi termodinamiki (Direct and Inverse Problems in Chemical Thermodynamics), Novosibirsk: Nauka, 1987, pp. 108–123.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by O. Tsarev
Rights and permissions
About this article
Cite this article
Denisova, L.T., Molokeev, M.S., Irtyugo, L.A. et al. Synthesis, Structure, and Thermophysical Properties of EuGaGe2O7 . Inorg Mater 56, 854–858 (2020). https://doi.org/10.1134/S002016852008004X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S002016852008004X