Abstract—
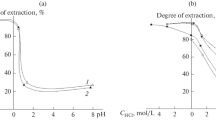
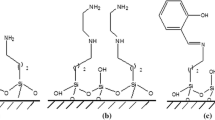
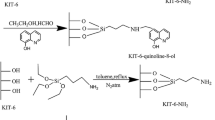
We have synthesized silica-based sorbents with an MSM-48 pore structure, modified with hydrazide and amide groups based on Versatic tret-carboxylic acids, and investigated their textural and structural properties. The kinetics of Cu(II), Ni(II), and Co(II) sorption on the sorbents from aqueous solutions have been studied, and the effects of the silica modification process and modifier concentration on the sorption capacity of the silicas for the three metals present together have been examined. The synthesized sorbents have been shown to be capable of separating the nonferrous metals studied.




Similar content being viewed by others
REFERENCES
Balakain, V.M., Dranitsina, N.V., Kholmanskaya, Yu.B., et al., New nitrogen- and phosphorus-containing ampholytes based on a polyacrylate matrix and copper, zinc, and iron sorption on them from sulfuric acid solutions, Zh. Prikl. Khim. (S.-Peterburg), 1981, vol. 54, no. 4, pp. 781–785.
Nikolaev, A.V., Fokin, A.V., Kolomiets, A.F., et al., Sorption of copper and nonferrous metals on sulfur-, nitrogen-, and sulfur/nitrogen-containing sorbents, Izv. Sib. Otd. Akad. Nauk SSSR,Ser. Khim. Nauk, 1977, vol. 4, no. 9, pp. 34–40.
Oskotskaya, E.P., Basargin, H.H., Ignatov, D.E., et al., Copper, cobalt, and nickel group preconcentration with a polymer chelate sorbent, Zav. Lab. Diagn. Mater., 1999, vol. 65, no. 3, pp. 10–14.
Kondrashova, N., Saenko, E., Lebedeva, I., Valtsifer, V., and Strelnikov, V., Effect of organic-silane additives on textural–structural properties of mesoporous silicate materials, Microporous Mesoporous Mater., 2012, vol. 153, pp. 275–281. https://doi.org/10.1016/j.micromeso.2011.12.017
Frolov, Yu.G., Kurs kolloidnoi khimii: poverkhnostnye yavleniya i dispersnye sistemy (A Course in Colloid Chemistry: Surface Phenomena and Disperse Systems), Moscow: Khimiya, 1988.
Batueva, T.D., Kondrashova, N.B., Kuz’micheva, N.D., et al., Physicochemical properties of mesoporous silicas modified with hydrazide and amide functional groups, Russ. J. Appl. Chem., 2017, vol. 90, no. 11, pp. 1746–1752. https://doi.org/10.1134/S1070427217110039
Tager, A.A., Fizikokhimiya polimerov (Physical Chemistry of Polymers), Moscow: Nauchnyi Mir, 2007.
Alosmanov, R.M., Kinetics of sorption of lead and zinc ions on a phosphorus-containing cation, Vestn. Mosk. Gos. Univ., Ser. 2:Khim., 2011, vol. 52, no. 2, pp. 145–148.
ACKNOWLEDGMENTS
In this study, we used equipment at the Materials and Matter Characterization Shared Research Facilities Center, Perm Federal Research Center, Ural Branch, Russian Academy of Sciences.
Funding
This work was supported by the Russian Federation Ministry of Science and Higher Education (state research target no. AAAA-A18-118032790022-7).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by O. Tsarev
Rights and permissions
About this article
Cite this article
Batueva, T.D., Kondrashova, N.B. & Shcherban’, M.G. Modified MCM-48 Mesoporous Materials and Their Sorption Capacity for Nonferrous Ions. Inorg Mater 56, 360–365 (2020). https://doi.org/10.1134/S0020168520040020
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0020168520040020