Abstract—
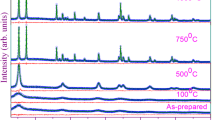
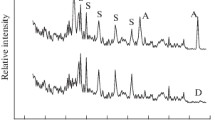
The metastable structure state of the anatase phase of titanium dioxide has been shown to be a favorable basis for a targeted influence on it with the aim of producing new oxide phases consisting of nanoparticles and, accordingly, having a new combination of properties. Mechanical activation and subsequent annealing of the activated powders result in a sequence of phase transformations (anatase–brookite–rutile), thereby ensuring structural ordering of titanium dioxide. Transformations of the titanium oxide phases have been studied in detail by X-ray diffraction. We have evaluated the crystallite (coherent scattering domain) size and internal strain (ε) from diffraction line broadening by profile fitting with the pseudo-Voigt function. The average particle size was 20 nm after mechanical activation, whereas heat treatment increased it by almost a factor of 2. Similar results have been obtained for internal strain. The pore system of the particles is formed by mesopores. The hysteresis loop observed in nitrogen sorption–desorption isotherms suggests that the titanium dioxide particles have platelike morphology. The results obtained in this study have been used to develop innovative technology of a special grade of titanium dioxide for the preparation of thermally stable glues with dielectric properties and high tensile and shear strength.







Similar content being viewed by others
REFERENCES
Guoma, P.I. and Mills, M.J., Anatase-to-rutile transformation in titania powders, J. Am. Ceram. Soc., 2001, vol. 84, no. 3, pp. 619–622. https://doi.org/10.1111/j.1151-2916.2001.tb00709
Salari, M., Rezaee, M., Mousavikoie, S.M., Marashi, P., and Aboutalebi, H., Effect of milling time on mechanochemical synthesis of TiO2 nanoparticles, Int. J. Mod. Phys. B, 2008, vol. 22, no. 18, pp. 2955–2961. https://doi.org/10.1142/S0217979208047808
Muscat, J., Swamy, V., and Harrison, N.M., First-principles calculations of the phase stability of TiO2, Phys. Rev. B: Condens. Matter Mater. Phys., 2002, vol. 65, paper 224 112. https://doi.org/10.1103/PhysRevB.65.224112
Zhang, H. and Banfield, J.F., Thermodynamic analysis of phase stability of nanocrystalline titania, J. Mater. Chem., 1998, vol. 8, no. 9, pp. 2073–2076. https://doi.org/10.1039/A802619J
Lu, H.M., Zhang, W.X., and Jiang, Q., Phase stability of nanoanatase, Adv. Eng. Mater., 2003, vol. 5, no. 11, pp. 787–788. https://doi.org/10.1002/adem.200300359
Zhang, H. and Banfield, J.F., Understanding polymorphic phase transformation behavior during growth of nanocrystalline aggregates: insights from TiO2, J. Phys. Chem. B, 2000, vol. 104, no. 15, pp. 3481–3487. https://doi.org/10.1021/jp000499j
Cheng, P., Zheng, M., Jin, Y., Huang, Q., and Gu, M., Preparation and characterization of silica-doped titania photocatalyst through sol–gel method, Mater. Lett., 2003, vol. 57, no. 20, pp. 2989–2994. https://doi.org/10.1016/S0167-577X(02)01409-X
Li, Y., White, T.J., and Lim, S.H., Low-temperature synthesis and microstructural control of titania nanoparticles, J. Solid State Chem., 2004, vol. 177, nos. 4–5, pp. 1372–1381. https://doi.org/10.1016/j.jssc.2003.11.016
Li, G.L. and Wang, G.H., Synthesis of nanometer-sized TiO2 particles by a microemulsion method, Nanostruct. Mater., 1999, vol. 11, no. 5, pp. 663–668. https://doi.org/10.1016/s0965-9773(99)00354-2
Tavangarian, F. and Emadi, R., Mechanochemical synthesis of single phase nanocrystalline forsterite powder, Int. J. Mod. Phys. B, 2010, vol. 24, no. 3, pp. 343–350. https://doi.org/10.1142/S0217979210053987
Raiender, G. and Giri, P.K., Strain induced phase formation, microstructural evolution and bandgap narrowing in strained TiO2 nanocrystals grown by ball milling, J. Alloys Compd., 2016, vol. 676, pp. 591–600. https://doi.org/10.1016/j.jallcom.2016.03.154
Gerasimova, L.G., Maslova, M.V., and Shchukina, E.S., The technology of sphene concentrate treatment to obtain titanium salts, Theor. Found. Chem. Eng., 2009, vol. 43, no. 4, pp. 464–467.
Rietveld, H., A profile refinement method for nuclear and magnetic structures, J. Appl. Crystallogr., 1969, vol. 2, no. 2, pp. 65–71.
Kozhevnikova, N.S., Kurlov, A.S., Uritskaya, A.A., and Rempel, A.A., Diffraction analysis of nanocrystalline particle size of lead and cadmium sulfides prepared by chemical deposition from aqueous solutions, J. Struct. Chem., 2004, vol. 45, no. 1, pp. S154–S159.
Kurlov, A.S. and Gusev, A.I., Determination of the particle sizes, microstrains, and degree of inhomogeneity in nanostructured materials from X-ray diffraction data, Glass. Phys. Chem., 2007, vol. 33, no. 3, pp. 276–282.
Valeeva, A.A., Petrovykh, K.A., Schroettner, Kh., and Rempel, A.A., Effect of stoichiometry on the size of titanium monoxide nanoparticles produced by fragmentation, Inorg. Mater., 2015, vol. 51, no. 11, pp. 1132–1137.
Williamson, G.K. and Hall, W.H., X-ray line broadening from filed aluminium and wolfram, Acta Metall., 1953, vol. 1, no. 1, pp. 22–31.
Mingming Wei, Li Zhang, Yongqiang Xiong, Jinhua Li, and Ping’an Peng, Nanopore structure characterization for organic-rich shale using the non-local-density functional theory by a combination of N2 and CO2 adsorption, Microporous Mesoporous Mater., 2016, vol. 227, pp. 88–94. https://doi.org/10.1016/j.micromeso.2016.02.050
Kurlov, A.S. and Gusev, A.I., Effect of ball milling parameters on the particle size in nanocrystalline powders, Tech. Phys. Lett., 2007, vol. 33, no. 10, pp. 828–832.
Lowell, S., Shields, J.E., Thomas, M.A., and Thommes, M., Characterization of Porous Solids and Powders: Surface Area, Pore Size and Density, Dordrecht: Kluwer, 2004.
Brunauer, S., Emmett, P.H., and Teller, E., Adsorption of gases in multimolecular layers, J. Am. Chem. Soc., 1938, vol. 60, no. 2, pp. 309–319.
Thommes, M., Kaneko, K., Neimark, A.V., Olivier, J.P., Rodriguez-Reinoso, F., Rouquerol, J., and Sing, K.S.W., Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC technical report), Pure Appl. Chem., 2015, vol. 87, nos. 9–10, pp. 1051–1069. https://doi.org/10.1515/pac-2014-1117
Vyacheslavov, A.S. and Efremova, M., Opredelenie ploshchadi poverkhnosti i poristosti materialov metodom sorbtsii gazov (Metodicheskaya razrabotka) (Determination of the Specific Surface Area and Porosity of Materials by Gas Sorption Measurements: A Methodological Guidance), Moscow: Mosk. Gos. Univ., 2011.
Kuz’mich, Yu.V. and Gerasimova, L.G., Effect of grinding media on the anatase-to-rutile phase transformation, Tr. Kol’sk. Nauchn. Tsentra Ross. Akad. Nauk, 2018, part 1, pp. 315–318.
Gerasimova, L.G., Kuz’mich, Yu.V., Nikolaev, A.I., Shchukina, E.S., and Kiselev, Yu.G., RF Patent 2 613 509, Byull. Izobret., 2017, no. 8.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by O. Tsarev
Rights and permissions
About this article
Cite this article
Kuz’mich, Y.V., Gerasimova, L.G. & Shchukina, E.S. Structural Transformations of TiO2 during Mechanical Activation and Subsequent Annealing. Inorg Mater 56, 156–163 (2020). https://doi.org/10.1134/S0020168520020090
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0020168520020090