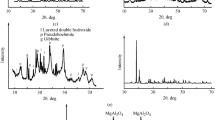
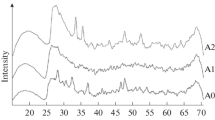
Abstract—
We have studied interaction between the microwave activation product of gibbsite and an aqueous cobalt nitrate solution at room temperature, atmospheric pressure, and pH 8.0 ± 0.3. The results demonstrate that, under such conditions, the reaction in the near-surface region of the microwave-activated gibbsite particles leads to the formation of an aluminum cobalt oxyhydroxide with fragments of spinel structure and the composition Со2.3Al0.7O4, and a Со6 –xAl2 –x(OH)1.5 · 3.5H2O (0 ≤ x ≤ 1) nonstoichiometric hydrotalcite. The morphology of such compounds is determined by 2D nanoparticles in the form of bent sheets 2–5 nm in thickness and 500 nm or more in length. After calcination at 500°C, the composition of the reaction products corresponds to the aluminum cobalt oxide Со2.3Al0.7O4 with the spinel structure.






Similar content being viewed by others
REFERENCES
Merikhi, J., Jungk, H., and Feldmann, C., Sub-micrometer CoAl2O4 pigment particles—synthesis and preparation of coatings, J. Mater. Chem., 2002, vol. 10, pp. 1311–1314.
Rangappa, D., Ohara, S., Naka, T., Kondo, A., Ishii, M., and Adschiri, T., Synthesis and organic modification of CoAl2O4 nanocrystals supercritical water conditions, J. Mater. Chem., 2007, vol. 17, pp. 4426–4429.
Evans, D.G. and Slade, R.C.T., Structural aspects of layered double hydroxides, Struct. Bonding, 2006, vol. 119, pp. 1–87.
Khodakov, A.Y., Chu, W., and Fongarland, P., Advances in the development of novel cobalt Fischer–Tropsch catalysts for synthesis of long-chain hydrocarbons and clean fuels, Chem. Rev., 2007, vol. 107, pp. 1692–1744.
Li, F. and Duan, X., Applications of layered double hydroxides, Struct. Bonding, 2006, vol. 119, pp. 193–223.
Tian, Li, Huang, K., Liu, Y., and Liu, S., Topotactic synthesis of Co3O4 nanoboxes from Co(OH)2 nanoflakes, J. Solid State Chem., 2011, vol. 184, pp. 2961–2965.
Tang, Y., Liu, Y., Yu, S., Mu, S., Xiao, S., Zhao, Y., and Gao, F., Morphology controlled synthesis of monodisperse cobalt hydroxide for supercapacitor with high performance and long cycle life, J. Power Sources, 2014, vol. 256, pp. 160–169.
Jacobs, G., Das, T.K., Zhang, Y., Li, J., Racoillet, G., and Davis, B.H., Fischer–Tropsch synthesis: support, loading, and promoter effects on the reducibility of cobalt catalysts, Appl. Catal., A, 2002, vol. 233, pp. 263–281.
Wang, C., Lui, S., Lui, L., and Bai, X., Synthesis of cobalt-aluminate spinels via glycine chelated precursors, J. Mater. Chem. Phys., 2006, vol. 96, pp. 361–370.
Li, W., Li, J., and Guo, J., Synthesis and characterization of nano crystalline CoAl2O4 spinel powder by low temperature combustion, J. Eur. Ceram. Soc., 2003, vol. 23, pp. 2289–2295.
Fedotov, M.A., Taraban, E.A., Krivoruchko, O.P., and Buyanov, R.A., Hydrolytic polycondensation of aqua ions in Al3+–Co2+ mixed nitrate solutions studied by nuclear magnetic resonance of different nuclei, Zh. Neorg. Khim., 1990, vol. 35, no. 5, pp. 1226–1230.
Taraban, E.A., Reaction between Al(III) and Co(II) hydroxides during mixing in an aqueous medium, Sib. Khim. Zh., 1991, no. 1, pp. 20–23.
Bai, C.S., Soled, S., Dwight, K., and Wold, A., Preparation and characterization of dispersed “cobalt oxide” supported on γ-Al2O3, J. Solid State Chem., 1991, vol. 91, no. 1, pp. 148–152.
Abdel-Salaman, K.M., Girgis, M.M., and Fahim, R.B., Surface texture of the mixed Al–Co oxide spinel phase, Surf. Technol., 1982, vol. 17, no. 4, pp. 281–290.
Fogg, A.M., Williams, G.R., Chester, R., and O’Hare, D.A., Novel family of layered double hydroxides—[MAl4(OH)12](NO3)2 · xH2O (M = Co, Ni, Cu, Zn), J. Mater. Chem., 2004, vol. 14, pp. 2369–2371.
Williams, G.R., Moorhouse, S.J., Timothy, J.P., Fogg, A.M., Rees, N.H., and O’Hare, D.A., New insights into the intercalation chemistry of Al(OH)3, Dalton Trans., 2011, vol. 40, pp. 6012–6022.
Krivoruchko, O.P., Buyanov, R.A., Paramzin, S.M., and Zolotovskii, B.P., Reactions of mechanochemically activated Al(III) hydroxides with crystalline divalent metal oxides, Kinet. Katal., 1988, vol. 29, no. 1, pp. 252–253.
Ragupathi, C., Vijaya, J.D., Narayanan, S., Jesudoss, S.K., and Kennedy, L.J., Highly selective oxidation of benzyl alcohol to benzaldehyde with hydrogen peroxide by cobalt aluminate catalysis: a comparison of conventional and microwave methods, Ceram. Int., 2015, vol. 41, pp. 2069–2080.
Moraz-Lazaro, J.P., Blanco, O., Rodriguez-Betancourtt, V.M., Reyes-Gomez, J., and Michel, C.R., Enhanced CO2-sensing response of nanostructured cobalt aluminate synthesized using a microwave-assisted colloidal method, Sens. Actuators, B, 2016, vol. 226, pp. 518–524.
Veronesi, P., Leonelli, C., and Bondioli, F., Energy efficiency in the microwave-assisted solid-state synthesis of cobalt aluminate pigment, Technologies, 2017, vol. 5, pp. 42–54.
Krivoruchko, O.P., Zhuzhgov, A.V., Khabibulin, D.F., Tanashev, Yu.Yu., Bolotov, V.A., Ishchenko, A.V., Molina, I.Yu., and Parmon, V.N., Unusual bulk amorphization of gibbsite into atomic size aluminum–oxygen complexes occurring within initial microcrystals under microwave radiation, Dokl. Phys. Chem., 2012, vol. 445, no. 5, pp. 128–133.
Zhuzhgov, A.V., Paukshtis, E.A., Krivoruchko, O.P., Molina, I.Yu., Larina, T.V., and Parmon, V.N., Regularities of Lewis site formation upon the microwave irradiation of gibbsite–γ-Al(OH)3, Russ. J. Phys. Chem. A, 2013, no. 9, pp. 1488–1497.
Ingram-Jones, V.J., Davies, R.C.T., Southern, J.C., and Salvador, S., Dehydroxylation sequences of gibbsite and boehmite: study of differences between soak and flash calcinations and of particle-size effects, J. Mater. Chem., 1996, vol. 6, no. 1, pp. 73–79.
Bolotov, V.A., Chernousov, Yu.D., Udalov, E.I., Tanashev, Yu.Yu., and Parmon, V.N., Microwave-driven high-temperature chemical reactions, Vestn. Nizhegorodsk. Gos. Univ., Ser.: Fiz., 2009, vol. 54, no. 2, pp. 78–83.
Gareth, R., O’Hare, W., and O’Hare, D., Towards understanding, control and application of layered double hydroxide chemistry, J. Mater. Chem., 2006, vol. 16, pp. 3065–3074.
Krivoruchko, O.P., Larina, T.V., Anufrienko, V.F., Molina, I.Yu., and Paukshtis, E.A., Synthesis, electronic state, and particle size stabilization of nanoparticulate [Co(OH)2 \(({{{\text{H}}}_{{\text{3}}}}{\text{O}})_{\delta }^{ + }\)]δ+, Inorg. Mater., 2009, vol. 45, no. 12, pp. 1355–1361.
Krivoruchko, O.P., Gavrilov, V.Yu., Molina, I.Yu., and Larina, T.V., Distribution of the cobalt-containing component in the pore space of HZSM-5 upon a postsynthetic modification of the zeolite with hydroxo compounds of Co2+, Kinet. Katal., 2008, vol. 49, no. 2, pp. 285–290.
Casado, P.G. and Rasines, I., The series of spinels Co3 – sAls (0< s <2): study of CoAl2O4, J. Solid State Chem., 1984, vol. 52, pp. 187–190.
ACKNOWLEDGMENTS
This work was supported by the Russian Federation Ministry of Education and Science (state research target for the Boreskov Institute of Catalysis, Siberian Branch, Russian Academy of Sciences, project no. AAAA-A17-117041710090-3).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by O. Tsarev
Rights and permissions
About this article
Cite this article
Zhuzhgov, A.V., Krivoruchko, O.P., Larina, T.V. et al. Synthesis of Highly Dispersed 2D Aluminum Cobalt Oxyhydroxide Compounds Based on Microwave-Activation Products of Crystalline Gibbsite. Inorg Mater 55, 380–389 (2019). https://doi.org/10.1134/S0020168519040162
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0020168519040162