Abstract—
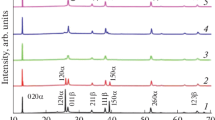
MoO3 samples have been prepared by vapor transport deposition under different process conditions: at two distinct hot-zone temperatures in a tube furnace (800 and 1100°C) and with different additives to argon as a major carrier gas: O2, H2O vapor, or N2O gas. According to X-ray diffraction, electron-microscopic, and optical characterization results, the layered structure of microcrystals and band gap of MoO3 are sensitive to not only vapor transport deposition conditions (synthesis temperature and vapor phase composition) but also mechanical treatment (grinding). At a synthesis temperature of 800°C, the distortion of the MoO3 crystal lattice by incorporated H2O molecules causes MoO3 to transform from the major, orthorhombic α-phase (space group Pbnm) into a monoclinic phase (P21/n), which is accompanied by a reduction in band gap from 2.85 to 2.68 eV. At a higher synthesis temperature of 1100°C, neither hydrogen or oxygen impurities originating from water (H2O) vapor nor nitrogen or oxygen impurities originating from N2O change the layered orthorhombic structure of the microcrystals, but the addition of N2O to the vapor transport medium reduces the band gap of MoO3 to 2.51 eV. Mechanical treatment (grinding) of the MoO3 microcrystals synthesized at the higher temperature (1100°C) with the addition of water vapor or N2O to argon carrier gas produces a monoclinic phase (space group P21/n) in addition to the major, stable, orthorhombic phase (Pbnm). The MoO3 microcrystals synthesized at a temperature of 800°C are more resistant to mechanical treatment: after grinding, they consist of only one phase: orthorhombic (Pbnm) in the case of synthesis in an argon–oxygen vapor transport medium or monoclinic (P21/n) in the case of the addition of water vapor to argon as a major carrier gas.







Similar content being viewed by others
REFERENCES
Goodenough, J.B., Chemistry and uses of molybdenum, Proc. Climax 4th Int. Conf., Barry H.F. and Mitchell P.C., Eds., Ann Arbor: Climax Molybdenum Corp., 1982. vol. 1, p. 1.
Pichat, P., Mozzanega, M.N., and Hoang-Van, C., Room temperature photoassisted formation of hydrogen molybdenum bronzes with alcohol as hydrogen source, Phys. Chem., 1988, vol. 92, pp. 464–467.
Erre, R., Legay, M.H., and Fripiat, J.J., Reaction of molecular hydrogen with the 100 face of MoO3: II. Kinetics initiated by atomic hydrogen and characterization of the surface electronic state, Surf. Sci., 1983, vol. 127, no. 1, pp. 69–82.
Deb, S. and Khoc, R., Physical properties of a transition metal oxide: optical and photoelectric properties of single crystal and thin film molybdenum trioxide, Proc. R. Soc. London, Ser. A, 1968, vol. 304, pp. 211–231. https://doi.org/10.1098/rspa
Goodenough, J.B., Progress in Solid State Chemistry, Reiss, H., Ed., London: Pergamon, 1971, vol. 5.
Halevi, P., Electromagnetic Waves, vol. 1: Spatial Dispersion in Solids and Plasmas, Halevi, P., Ed., Amsterdam: Elsevier, 1992.
Andersson, G. and Magneli, A., On the crystal structure of molybdenum trioxide, Acta Chem. Scand., 1950, vol. 4, pp. 793–797. https://doi.org/10.3891/acta.chem.scand.04-0793
Negishi, H., Negishi, S., Kuroiwa, Y., Sato, N., and Aoyagi, S., Anisotropic thermal expansion of layered MoO3 crystals, Phys. Rev. B: Condens. Matter Mater. Phys., 2004, vol. 69, paper 064 111. https://doi.org/10.1103/PhysRevB.69.064111
Yao, J., Hashimoto, K., and Fujishima, A., Photochromism induced in an electrolytically pretreated MoO3 thin film by visible light, Nature, 1992, vol. 355, pp. 624–626. https://doi.org/10.1038/355624a0
Bechinger, C., Ferrere, S., Zaban, A., Sprague, J.B., and Gregg, A., Photoelectrochromic windows and displays, Nature, 1996, vol. 383, pp. 608–613.
Hu, X.K., Qian, Y.T., Song, Z.T., Huang, J.R., Cao, R., and Xiao, J.Q., Comparative study on MoO3 and HxMoO3 nanobelts, Chem. Mater., 2008, vol. 20, pp. 1527–1533.
Balendhran, S., Walia, S., Nili, H., and Kalantar-zadeh, K., Advances in two-dimensional molybdenum trioxide and dichalcogenides, Funct. Mater., 2013, vol. 23, pp. 3952–3970.
Huang, P.R., He, Y., Cao, C., and Lu, Z.H., Impact of lattice distortion and electron doping on α-MoO3 electronic structure, Sci. Rep., 2014, vol. 4, paper 7131. https://doi.org/10.1038/srep07131
Gusev, A.I., Nanokristallicheskie materialy, metody polucheniya i svoistva (Nanocrystalline Materials: Preparation and Properties), Yekaterinburg: Ural. Otd. Ross. Akad. Nauk, 1998.
Rempel’, A.A. and Valeeva, A.A., Materialy i metody nanotekhnologii (Materials and Methods in Nanotechnologies), Yekaterinburg: Ural. Univ., 2015.
Kotsareva, K.V., Synthesis and morphology of hybrid nanosystems based on graphene and Ni, Co, Mo, W, and Si oxides, Cand. Sci. (Chem.) Dissertation, Moscow, 2017.
Joint Committee for Powder Diffraction Standards–International Center for Diffraction Data, PDF card 2012 (00-005-0508).
Joint Committee for Powder Diffraction Standards–International Center for Diffraction Data, PDF card 2012 (00-005-1513).
Kortum, G., Reflectance Spectroscopy, New York: Springer, 1969.
Tauc, J., Grigorovici, R., and Vancu, A., Optical properties and electronic structure of amorphous germanium, Phys. Status Solidi, 1966, vol. 15, pp. 627–637. https://doi.org/10.1002/pssb.19660150224
Tauc, J., Optical properties and electronic structure of amorphous Ge and Si, Mater. Res. Bull., 1968, vol. 3, pp. 37–46.
Lu, J., Sun, Ch., Zheng, M., Wang, Y., Nripan, M., van Kan, J.A., Mhaisalkar, S.G., and Sow, C.H., Bandgap engineering of phosphorene by laser oxidation toward functional 2D materials, J. Phys. Chem. C, 2012, vol. 116, pp. 22 015–22 020.
Mestl, G.N., Verbruggen, F.D., and Knozinger, H., Mechanically activated MoO3. Characterization of defect structures, Langmuir, 1995, vol. 11, pp. 3035–3041.
Surovoi, E.P. and Eremeeva, G.O., Thermally stimulated transformations of molybdenum(VI) oxide nanofilms, Polzunovskii Vestn., 2011, vol. 4-1, pp. 142–146.
ACKNOWLEDGMENTS
This work was supported by the Russian Federation Ministry of Education and Science (state research target for higher education institutions in the field of scientific activity in 2017–2019, project nos. 3.6263.2017/VU and 16.8158.2017/8.9).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by O. Tsarev
Rights and permissions
About this article
Cite this article
Domashevskaya, E.P., Ivkov, S.A., Dambos, aH.H. et al. Effect of Process Conditions on the Structure and Optical Properties of MoO3 Produced by Vapor Transport Deposition. Inorg Mater 55, 49–58 (2019). https://doi.org/10.1134/S0020168519010035
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0020168519010035