Abstract
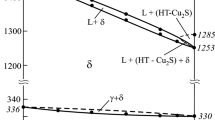
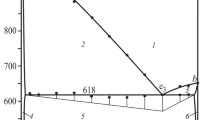
The Cu2Se–Cu3AsSe4–Se system has been studied using differential thermal analysis, X-ray diffraction, and emf measurements on concentration cells using Cu4RbCl3I2 as a solid electrolyte. We have constructed a number of vertical sections through the phase diagram, the room-temperature solid-state phase compatibility diagram, and a projection of the liquidus surface. The primary crystallization fields of the phases present and the types and coordinates of in- and univariant equilibria in the system have been identified. The system has been shown to contain a broad liquid–liquid immiscibility region. Using emf data, we evaluated the standard thermodynamic functions of formation and standard entropy of the Cu3AsSe4 compound.
Similar content being viewed by others
References
Dvoinye i mnogokomponentnye sistemy na osnove medi: Spravochnik (Binary and Multicomponent Copper Systems: A Handbook), Abrikosov, N.Kh., Ed., Moscow: Nauka, 1979.
Lazarev, V.B., Berul’, S.I., and Salov, A.V., Troinye poluprovodnikovye soedineniya v sistemakh AI–BV–CVI (Ternary Semiconductors in I–V–VI Systems), Moscow: Nauka, 1982.
Babanly, M.B., Yusibov, Yu.A., and Abishev, V.T., Trekhkomponentnye khal’kogenidy na osnove medi i serebra (Ternary Copper and Silver Chalcogenides), Baku: Bakinsk. Gos. Univ., 1993.
Avellaneda, D., Nair, M.T.S., and Nair, P.K., Cu2SnS3 and Cu4SnS4 thin films via chemical deposition for photovoltaic application, J. Electrochem. Soc., 2010, vol. 157, no. 6, pp. D346–D352.
Marcano, G., Bracho, D.B., Rincon, C., Perez, G.S., and Nieves, L., On the temperature dependence of the electrical and optical properties of Cu2GeSe3, J. Appl. Phys., 2000, vol. 88, pp. 822–828.
Morelli, D.T. and Skoug, E.J., Ternary copper-based diamond-like semiconductors for thermoelectric applications, 2009 MRS Spring Meet.: Materials and Devices for Thermal-to-Electric Energy Conversion, San Francisco, 2009, vol. 1166, pp. 171–176.
Ivanov-Schitz, A.K. and Murin, I.V., Ionika tverdogo tela (Solid State Ionics), St. Petersburg: S.-Peterburg. Univ., 2000, vol.1.
Babanly, M.B., Yusibov, Y.A., and Babanly, N.B., The EMF method with solid-state electrolyte in the thermodynamic investigation of ternary copper and silver chalcogenides, Electromotive Force and Measurement in Several Systems, Kara, S., Ed., InTech, 2011, pp. 57–78.
Popesku, M.A., Non-crystalline Chalcogenides, Berlin: Springer, 2001.
Xin, S., Liu, J., and Salmon, P., Structure of Cu–As–Se glasses investigated by neutron diffraction with copper isotope substitution, Phys. Rev. B: Condens. Matter Mater. Phys., 2008, vol. 78, no. 6, paper 064207.
Tédenac, J.-C., Arsenic–copper–selenium, Ternary Alloys, Petzow, G., Ed., Weinheim: VCH, 1994, vol. 10, pp. 129–134.
Cohen, K., Rivet, J., and Dugue, J., Description of the Cu–As–Se ternary system, J. Alloys Compd., 1995, vol. 224, pp. 316–329.
Binary Alloy Phase Diagrams, Massalski, T.B., Ed., Materials Park: ASM International, 1990, 2nd ed.
Diagrammy sostoyaniya dvoinykh metallicheskikh sistem. Spravochnik (Phase Diagrams of Binary Metallic Systems: A Handbook), Lyakishev, N.P., Ed., Moscow: Mashinostroenie, 1997, vol.2.
Blachnik, R. and Gather, B., Enthalpies of melting of some ternary ABX2-compounds (A = Cu, Ag; B = As, Sb, Bi; X = S, Se, Te), Z. Naturforsch., B: Chem. Sci., 1972, vol. 27, no. 11, pp. 1417–420.
Potorii, M.V., Preparation and physicochemical properties of ternary selenides in I–V–VI systems, Extended Abstract of Cand. Sci. (Chem.) Dissertation, Lvov: Lvov State Univ., 1980.
Emsley, J., The Elements, Oxford: Clarendon, 1991
Glazov, V.M., Burkhanov, A.S., and Saleeva, N.M., A procedure for the preparation of single-phase copper and silver chalcogenides, Izv. Akad. Nauk SSSR, Neorg. Mater., 1977, vol. 13, no. 5, pp. 917–919.
Morachevskii, A.G., Voronin, G.F., Geiderikh, V.A., and Kutsenok, I.B., Elektrokhimicheskie metody issledovaniya v termodinamike metallicheskikh sistem (Electrochemical Methods of Investigation in Thermodynamics of Metal Systems), Moscow: ITsK Akademkniga, 2003.
Babanly, M.B., Gasanova, Z.T., Mashadieva, L.F., Zlomanov, V.P., and Yusibov, Yu.A., Thermodynamic study of the Cu–As–S system by emf measurements with Cu4RbCl3I2 as a solid electrolyte, Inorg. Mater, 2012, vol. 48, no. 3, pp. 225–228.
Babanly, N.B., Salimov, Z.E., Akhmedov, M.M., and Babanly, M.B., Thermodynamic study of Cu–Tl–Te system using emf technique with Cu4RbCl3I2 solid electrolyte, Russ. J. Electrochem., 2012, vol. 48, no. 1, pp. 68–73.
Babanly, M.B. and Yusibov, Yu.A., Elektrokhimicheskie metody v termodinamike neorganicheskikh sistem (Electrochemical Methods in Thermodynamics of Inorganic Systems), Baku: ELM, 2011.
Babanly, N.B., Yusibov, Yu.A., Aliev, Z.S., and Babanly, M.B., Phase equilibria in the Cu–Bi–Se system and thermodynamic properties of copper selenobismuthates, Russ. J. Inorg. Chem., 2010, vol 55, no. 9, pp. 1471–1481.
Babanly, N.B., Aliev, Z.S., Yusibov, Yu.A., and Babanly, M.B., A Thermodynamic study of Cu–Tl–S system by emf method with Cu4RbCl3I2 solid electrolyte, Russ. J. Electrochem., 2010, vol. 46, no. 3, pp. 354–358.
Abbasov, A.S., Azizov, T.Kh., Aliev, I.Ya., and Mustafaev, F.M., Thermodynamic study of III–V, III-VI, and I–VI semiconductor systems, in Termodinamicheskie svoistva metallicheskikh sistem (Thermodynamic Properties of Metallic Systems), Baku: Elm, 1975, pp. 404–416.
Baza dannykh “Termicheskie konstanty veshchestv”. Elektronnaya versiya (Thermal Constants of Substances Database, Electronic Version), Iorish, V.S. and Yungman, V.S., Eds., 2006. http://www/chem.msu.su/cgibin/ tkv.
Kubaschewski, O., Alcock, C.B., and Spencer, P.J., Materials Thermochemistry, Oxford: Pergamon, 1993.
Babanly, D.M., Velieva, G.M., Imamaliyeva, S.Z., and Babanly, M.B., Thermodynamic functions of arsenic selenides, Russ. J. Phys. Chem. A, 2017, vol. 91, no. 7, pp. 1170–1173.
O’Hare, P.A.G., Calorimetric measurements of the specific energies of reaction of arsenic and of selenium with fluorine. Standard molar enthalpies of formation at the temperature 298.15 K of AsF5, SeF6, As2Se3, As4S4, and As2S3, J. Chem. Thermodyn., 1993, vol. 25, no. 2, pp. 391–402.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © L.F. Mashadieva, Z.T. Gasanova, Yu.A. Yusibov, M.B. Babanly, 2018, published in Neorganicheskie Materialy, 2018, Vol. 54, No. 1, pp. 11–18.
Rights and permissions
About this article
Cite this article
Mashadieva, L.F., Gasanova, Z.T., Yusibov, Y.A. et al. Phase Equilibria in the Cu2Se–Cu3AsSe4–Se System and Thermodynamic Properties of Cu3AsSe4. Inorg Mater 54, 8–16 (2018). https://doi.org/10.1134/S0020168518010090
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0020168518010090