Abstract
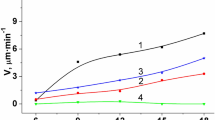
We have studied the chemical dissolution of InAs, InSb, GaAs, and GaSb crystals in (NH4)2Cr2O7–HBr–C6H8O7 solutions. The dissolution rate of the crystals has been measured as a function of etchant composition, and the kinetics of the chemical interaction of the semiconductors with solutions have been investigated in detail. The dissolution rate has been shown to be diffusion-limited. Citric acid helps to reduce the etch rate and improves the polishing performance of the etching solutions.
Similar content being viewed by others
References
Bulygina, E.V., Makarchuk, V.V., Panfilov, Yu.V., et al., Nanorazmernye struktury: klassifikatsiya, formirovanie i issledovanie (Nanostructures: Classification, Formation, and Characterization), Moscow: Sains-Press, 2006.
Kusyak, N.V., Tomashyk, Z.F., and Tomashyk, V.M., Liquid-phase etching of indium antimonide with bromine- releasing H2O2–HBr–organic acid solutions, Nov. Tekhnol., 2005, vol. 3, no. 9, pp. 12–16.
Tomashyk, Z.F., Shelyuk, I.A., Tomashyk, V.N., et al., Dynamic chemical polishing of GaAs, GaSb, InAs, and InSb crystals with H2O2–HBr–lactic acid etchants, Vopr. Khim. Khim. Tekhnol., 2009, no. 5, pp. 117–120.
Bryce, C. and Berk, D., A kinetic study of gallium arsenide in H2O2–NH4OH–H2O solutions, Ind. Eng. Chem. Res., 1996, vol. 35, no. 12, pp. 4464–4470.
Kusyak, N.V., Tomashyk, Z.F., Tomashyk, V.N., et al., Dissolution of indium arsenide and indium antimonide in the K2Cr2O7–HBr–HCl–H2O system, Ukr. Khim. Zh., 2002, vol. 68, no. 1, pp. 11–14.
Tomashik, Z.F., Kusiak, N.V., Tomashik, V.N., et al., Polishing of InSb in K2Cr2O7–HBr–HCl (oxalic acid) solutions, Proc. SPIE, 2001, vol. 4355, pp. 294–298.
Tomashik, Z.F., Kusyak, N.V., Tomashik, V.N., et al., Chemical etching of indium arsenide with K2Cr2O7–HBr–oxalic acid solutions, Kondens. Sredy Mezhfaznye Granitsy, 2001, vol. 3, no. 1, pp. 14–17.
Perevoshchikov, V.A., Dynamic chemical polishing of semiconductor surfaces, Vysokochist. Veshchestva, 1995, no. 2, pp. 5–29.
Novik, F.S., Planirovanie eksperimenta na simplekse pri izuchenii metallurgicheskikh sistem (Simplex Design of Experiments in Studies of Metallurgical Systems), Moscow: Metallurgiya, 1985.
Pop, S.S. and Sharodi, I.S., Fizychna Elektronika (Physical Electronics), Lviv: Evrosvit, 2001.
Perevoshchikov, V.A. and Gusev, V.K., Hydrodynamic conditions of the chemical polishing of semiconductor wafers, Zh. Prikl. Khim. (S.-Peterburg), 1970, vol. 43, no. 6, pp. 1238–1245.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © I.V. Levchenko, I.B. Stratiychuk, V.N. Tomashyk, G.P. Malanych, A.S. Stanetskaya, A.A. Korchevoi, 2017, published in Neorganicheskie Materialy, 2017, Vol. 53, No. 11, pp. 1137–1142.
Rights and permissions
About this article
Cite this article
Levchenko, I.V., Stratiychuk, I.B., Tomashyk, V.N. et al. Chemical interaction of InAs, InSb, GaAs, and GaSb crystal surfaces with (NH4)2Cr2O7–HBr–citric acid etching solutions. Inorg Mater 53, 1109–1114 (2017). https://doi.org/10.1134/S002016851711005X
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S002016851711005X