Abstract
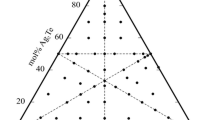
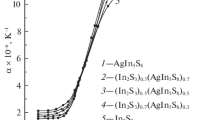
We have studied the formation of crystalline and glassy alloys in the Ag3GeS3Br-GeS2 system (0–52 mol % GeS2; Ag3GeS3Br is a glass-forming phase of variable composition) and determined the crystallographic parameters of the Ag3.179(9)Ge1.474(5)S4Br alloy as the saturated solid solution of GeS2 in Ag3GeS3Br: sp. gr. P213, a = 10.16572(3) Å, Z = 4. The electrical conductivity of the crystalline and glassy alloys was measured in the temperature range 250–410 K by a dc probe technique. The current carriers in the alloys are silver cations and halogen anions. We obtained materials with superionic conductivity and proposed a model for conduction in the alloys.
Similar content being viewed by others
References
Wagener, M., Deiseroth, H.-J., and Reiner, C., Ag6GeS4X2 (X: Cl, Br): Surprisingly no filled Laves phases but the first representatives of a new structure type, Z. Kristallogr., 2006, vol. 221, nos. 5–7, pp. 533–538.
Moroz, M.V., Demchenko, P.Yu., Akselrud, L.G., et al., Phase relation along the Ag8GeS6-[(AgBr)4 · GeS2] cross-section. Crystal structure and electric conductivity of Ag6GeS4Br2 in bulk, Chem. Met. Alloys, 2010, no. 3, pp. 161–168.
Laqibi, M., Cros, B., Peytavin, S., and Ribes, M., New silver superionic conductors Ag7XY5Z (X = Si, Ge, Sn; Y = S, Se; Z = Cl, Br, I)—synthesis and electrical studies, Solid State Ionics, 1987, vol. 23, nos. 1–2, pp. 21–26.
Ivanov-Shitz, A.K. and Murin, I.V., Ionika tverdogo tela (Solid State Ionics), St. Petersburg: S.-Peterburg. Univ., 2000, vol. 1.
Parthe, E., Contribution to the crystal chemistry of normal and defect tetrahedral structures, Z. Kristallogr., B, 1963, vol. 119, nos. 3–4, pp. 204–225.
Berger, L.I. and Prochukhan, V.D., Troinye almazopodobnye poluprovodniki (Ternary Diamond-Like Semiconductors), Moscow: Metallurgiya, 1968.
Goryunova, N.A., Slozhnye almazopodobnye poluprovodniki (Multicomponent Diamond-Like Semiconductors), Moscow: Sovetskoe Radio, 1968.
Roos, J., Brinkmann, D., Mali, M., et al., (AgI)x · (Ag2S · GeS2)1 − x glasses studied by 109Ag NMR, Solid State Ionics, 1988, vols. 28–30, no. 1, pp. 710–712.
Kraus, W. and Nolze, G., PowderCell for Windows (Version 2.3), Berlin: Federal Inst. for Materials Research and Testing, 1999.
Stoe WinX POW, Version 2.21, Darmstadt: Stoe & Cie GmbH, 2007.
Mykolaychuk, A.G., Moroz, N.V., Demchenko, P.Yu., et al., Electrical conductivity of Ag8SnS6-Ag2SnS3-AgBr alloys, Inorg. Mater., 2010, vol. 46, no. 7, pp. 707–710.
Moroz, M.V., Demchenko, P.Yu., Akselrud, L.G., et al., Synthesis, structure and electrical properties of the new representative of argyrodite family, Ag3GeS3Br, 17th Conf. on Solid Compounds of Transition Elements, Annecy, 2010, p. 69.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © M.V. Moroz, P.Yu. Demchenko, O.G. Mykolaychuk, L.G. Akselrud, R.E. Gladyshevskii, 2013, published in Neorganicheskie Materialy, 2013, Vol. 49, No. 9, pp. 931–936.
Rights and permissions
About this article
Cite this article
Moroz, M.V., Demchenko, P.Y., Mykolaychuk, O.G. et al. Synthesis and electrical conductivity of crystalline and glassy alloys in the Ag3GeS3Br-GeS2 system. Inorg Mater 49, 867–871 (2013). https://doi.org/10.1134/S0020168513090100
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0020168513090100