Abstract
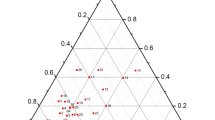
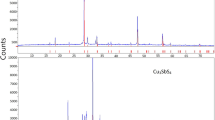
The Cu-Tl-Se system has been studied at temperatures from 300 to 420 K using emf measurements with Cu4RbCl3I2 as a Cu+ ion conducting solid electrolyte. The emf data have been used to map out the subsolidus phase diagram of the Cu-Tl-Se system in the composition region Tl2Se-CuTlSe-CuSe-Se. We have calculated the partial molar thermodynamic functions of the copper in the alloys and the standard thermodynamic functions of formation and standard entropies of the ternary compounds CuTlSe2, CuTlSe, and Cu2TlSe2. The results confirm that the thermodynamic properties of copper-containing ternary systems can be studied using the approach in question even when they contain an element (thallium in this study) located to the left of copper in the electrochemical series.
Similar content being viewed by others
References
Shevel’kov, A.V., Chemical Aspects of Thermoelectric Materials Engineering, Usp. Khim., 2008, vol. 77, no. 1, pp. 3–21.
Babanly, M.B., Yusibov, Yu.A., and Abishev, V.T., Trekhkomponentnye khal’kogenidy na osnove medi i serebra (Ternary Copper Silver Chalcogenides), Baku: BGU, 1993.
Makovicky, E., Crystal Structure of Sulphides and Other Chalcogenides, Rev. Mineral. Geochem., 2006, vol. 61, no. 1, pp. 7–125.
Abdel, A.A., Sharaf, K.A., and Elshafie, A., Some Physical Properties of CuTlSe Alloy, J. Mater. Sci. Technol., 2001, vol. 17, no. 2, pp. 229–232.
Kurosaki, K., Goto, K., Kosuga, A., et al., Thermoelectric and Thermophysical Characteristics of Cu2Te-Tl2Te Pseudo Binary System, Mater. Trans., 2006, vol. 47, no. 6, pp. 1432–1435.
Matsumoto, H., Kurosaki, K., Muta, H., and Yamanaka, S., Thermoelectric Properties of TlCu3Te2 and TlCu2Te2, J. Electron. Mater., 2009, vol. 38, no. 7, pp. 1350–1353.
Babanly, M.B., Yusibov, Yu.A., and Abishev, V.T., Metod EDS v termodinamike slozhnykh poluprovodnikovykh veshchestv (EMF Measurements in Thermodynamic Studies of Multicomponent Semiconductors), Baku: Bakinsk. Gos. Univ., 1992.
Babanly, N.B., Mokhtasebzade, Z., Aliev, I.I., and Babanly, M.B., Phase Equilibria in the Cu-Bi-Te System and Thermodynamic Properties of CuBiTe2, Dokl. Nats. Akad. Nauk Azerb., 2007, vol. 63, no. 1, pp. 41–54.
Babanly, N.B., Yusibov, Yu.A., Mirzoeva, R.J., et al., Cu4RbCl3I2 Solid Superionic Conductor in Thermodynamic Study of Three-Component Copper Chalcogenides, Russ. J. Electrochem., 2009, vol. 45, no. 4, pp. 405–410.
Babanly, N.B., Aliev, Z.S., Yusibov, Yu.A., and Babanly, M.B., A Thermodynamic Study of Cu-Tl-S System by EMF Method with Cu4RbCl3I2 Solid Electrolyte, Russ. J. Electrochem., 2010, vol. 46, no. 3, pp. 354–358.
Babanly, M.B., Yusibov, Y.A., Babanly, N.B., and Mashadiyeva, L.F., Phase Diagrams and Thermodynamic Properties of the Cu-BIV(BV)-Chalcogen Systems, VI Int. School-Conf. Phase Diagrams in Material Science, Kiev, 2001, pp. 5–6.
Ivanov-Schitz, A.K. and Murin, I.V., Ionika tverdogo tela (Solid State Ionics), St. Petersburg: S.-Peterburg. Univ., 2000, vol. 1.
Abishov, V.T., Babanly, M.B., and Kuliev, A.A., Phase Equilibria in the System Cu2Se-Tl2Se, Izv. Akad. Nauk SSSR, Neorg. Mater., 1979, vol. 15, no. 11, pp. 1926–1928.
Babanly, M.B., Physicochemical Principles of the Preparation and Thermodynamics of Ternary Thallium Chalcogenides, Doctoral (Chem.) Dissertation, Moscow: Moscow State Univ., 1987.
Voroshilov, Yu.V., Evstigneeva, T.L., and Nekrasov, I.Ya., Kristallokhimicheskie tablitsy troinykh khal’kogenidov (Crystal-Chemical Tables for Ternary Chalcogenides), Moscow: Nauka, 1989.
Abishov, V.T., Babanly, M.B., and Kuliev, A.A., Crystal Lattice of Cu(Ag)TlX and Phase Equilibria in the Cu(Ag)TlS-Cu(Ag)TlSe Systems, Izv. Vyssh. Uchebn. Zaved., Khim. Khim. Technol., 1981, vol. 24, no. 8, p. 931.
Binary Alloy Phase Diagrams, Massalski, T.B., Ed., Materials Park: ASM International, 1990, 2nd ed.
Doerffel, K., Statistik in der analytischen Chemie, Leipzig: Grundstoffindustrie, 1990.
Kornilov, A.N., Stepina, L.B., and Sokolov, V.A., Recommendations on Compact Representation of Experimental Data in Reports on Thermochemical and Thermodynamic Studies, Zh. Fiz. Khim., 1972, vol. 46, no. 11, pp. 2974–2979.
Kubaschewski, O., Alcock, C.B., and Spencer, P.J., Materials Thermochemistry, Oxford: Pergamon, 1993, 6th ed.
Baza dannykh termicheskie konstanty veshchestv. Elektronnaya versiya (Thermal Constants of Substances Database), Yungman, V.S., Ed., 2006: http://www.chem.msu.su/cgi-bin/tkv.
Vasil’ev, V.P., Nikol’skaya, A.V., and Gerasimov, Ya.I., Thermodynamic Study of Thallium-Selenium Alloys Using EMF Measurements, Zh. Neorg. Khim., 1971, vol. 45, no. 8, pp. 2061–2063.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © N.B. Babanly, 2011, published in Neorganicheskie Materialy, 2011, Vol. 47, No. 12, pp. 1433–1437.
Rights and permissions
About this article
Cite this article
Babanly, N.B. Thermodynamic properties of ternary phases in the Cu-Tl-Se system. Inorg Mater 47, 1306–1310 (2011). https://doi.org/10.1134/S0020168511120016
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0020168511120016