Abstract
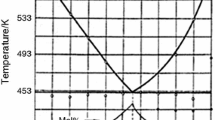
Subsolidus (500–700°C) phase relations in the K2O-MgO-B2O3 system have been studied by X-ray diffraction. The results are used to construct the phase compatibility diagram of the K2O-MgO-B2O3 system in the range 650–700°C. A new ternary compound of composition K2Mg3B2O7, with the constituent oxides in the molar ratio 1: 3: 1, is identified.
Similar content being viewed by others
References
Takamoto Sasaki, Yusuke Mori, Masashi Yoshimura, et al., Recent Development of Nonlinear Optical Borate Crystals: Key Materials for Generation of Visible and UV Light, Mater. Sci. Eng., 2000, vol. 30, pp. 1–54.
Furetta, C., Prokic, M., Salamon, R., et al., Applied Radiation and Isotopes Dosimetric Characterisation of a New Production of MgB4O7:Dy,Na Thermoluminescent Material, Appl. Radiat. Isot., 2000, vol. 52, pp. 243–250.
Mirjana Prokic, Lithium Borate Solid TL Detectors, Radiat. Meas., 2001, vol. 33, pp. 393–396.
Mirjana Prokic, Effect of Lithium Co-Dopant on the Thermoluminescence Response of Some Phosphors, Appl. Radiat. Isot., 2000, vol. 52, pp. 97–103.
Toropov, N.A. and Konovalov, P.F., Binary System MgO-B2O3, Zh. Fiz. Khim., 1940, vol. 14, pp. 1103–1109.
Davis, H.M. and Knight, M.A., The System Magnesium Oxide-Boric Oxide, J. Am. Ceram. Soc., 1945, vol. 28, pp. 97–102.
Kuzel, H.J., Crystal Structure of MgB4O7, Neues Jahrb. Mineral., Monatsh., 1964, no. 12, pp. 357–360.
Miyagawa, S., Ilirano, S., and Somiya, S., US-Japan Seminar on Basic Science of Ceramics, Equilibria and Kinetics in Modern Ceramic Processing. Phase Diagrams for Ceramics, J. Am. Ceram. Soc., 1981, vol. 4, pp. 99–105.
Kaplun, A.B. and Meshalkin, A.B., Phase Equilibria in the K2O-B2O3 System, Zh. Neorg. Khim., 2002, vol. 47, pp. 1167–1072.
Skorikova, A.V., Kargin, Yu.F., and Skorikov, V.M., Solidus Phase Relations in the K2O-Bi2O3-B2O3 System, Zh. Neorg. Khim., 2005, vol. 50, pp. 1851–1854.
Author information
Authors and Affiliations
Additional information
Original Russian Text © R.V. Kurbatov, B.G. Bazarov, A.K. Subanakov, Zh.G. Bazarova, 2010, published in Neorganicheskie Materialy, 2010, Vol. 46, No. 2, pp. 190–192.
Rights and permissions
About this article
Cite this article
Kurbatov, R.V., Bazarov, B.G., Subanakov, A.K. et al. Phase equilibria in the K2O-MgO-B2O3 system. Inorg Mater 46, 151–153 (2010). https://doi.org/10.1134/S0020168510020111
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0020168510020111