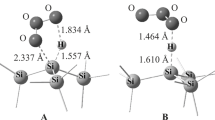
Abstract
The yield of singlet oxygen (1O2) due to the degradation of triethylsilane, dimethylphenylsilane, triphenylsilane, and dimethyl(trimethylsiloxy)silane hydrotrioxides has been determined for the first time using the IR chemiluminescence technique. The most effective sources of singlet oxygen in this series are triphenylsilyl hydrotrioxide and dimethyl(trimethylsiloxy)silyl hydrotrioxide. The yield of 1O2 upon their degradation is 69 and 92%, respectively.

Similar content being viewed by others
REFERENCES
Miyata, Sh., Yamada, T., Isozaki, T., Sugimura, H., Xu, Ya.-Z., and Suzuki, T., Photochem. Photobiol., 2018, vol. 94, no. 4, p. 677. https://doi.org/10.1111/php.12900
Westberg, M., Bregnhøj, M., and Etzerodt Ogilby, P.R., J. Phys. Chem. B, 2017, vol. 121, no. 12, p. 2561. https://doi.org/10.1021/acs.jpcb.7b00561
Solovieva, A.O., Kirakci, K., Ivanov, A.A., Kubát, P., Pozmogova, T.N., Miroshnichenko, S.M., Vorontsova, E.V., Chechushkov, A.V., Trifonova, K.E., Fufaeva, M.S., Kretov, E.I., Mironov, Yu.V., Poveshchenko, A.F., Lang, K., and Shestopalov, M.A., Inorg. Chem., 2017, vol. 56, no. 21, p. 13491. https://doi.org/10.1021/acs.inorgchem.7b02212
Prado, F.M., Oliveira, M.C.B., Miyamoto, S., Martinez, G.R., Medeiros, M.H.G., Ronsein, G.E., and Di Mascio, P., Free Radical Biol. Med., 2009, vol. 47, no. 4, p. 401. https://doi.org/10.1016/j.freeradbiomed.2009.05.001
Fudickar, W. and Linker, T., Org. Chem., 2017, vol. 82, no. 17, p. 9258. https://doi.org/10.1021/acs.joc.7b01765
Jary, W.G., Ganglberger, T., Pöchlauer, P., and Falk, H., Monatsh. Chem., 2005, vol. 136, no. 4, p. 537. https://doi.org/10.1007/s00706-004-0251-1
Cominade, A., Khatib, F., and Koenig, M., Can. J. Chem., 1985, vol. 63, p. 3203.
Ouellette, R.J. and Marks, D.L., J. Organomet. Chem., 1968, vol. 11, p. 407.
Spialter, L., Pazdernik, L., Bernstein, S., Swansiger, W.A., Buell, G.R., and Freeburger, M.E., Adv. Chem. Ser., 1972, vol. 112, p. 65.
De Meijere, A. and Wolf, F., Methoden der organische Chemie, Kropf, H., Ed., Stuttgart: Wiley, 1988, E13 Teil 1, p. 971.
Plesničar, B., Organic Polyoxides, Ando, W., Ed., Chichester: Wiley, 1992, p. 479.
Plesničar, B., Cerkovnik, J., Koller, J., and Kovač, F., J. Am. Chem. Soc., 1991, vol. 113, no. 13, p. 4946. https://doi.org/10.1021/ja00013a034
Cerkovnik, J., Tuttle, T., Kraka, E., Lendero, N., Plesničar, B., and Cremer, D., J. Am. Chem. Soc., 2006, vol. 128, no. 12, p. 4090. https://doi.org/10.1021/ja058065v
Corey, M.E.J., Mehrotra, M., and Khan, A.U., J. Am. Chem. Soc., 1986, vol. 108, no. 9, p. 2472. https://doi.org/10.1021/ja00269a070
Wu, H., Song, Q., Ran, G., Lu, X., and Xu, B., Trends Anal. Chem., 2011, vol. 30, no. 1, p. 133. https://doi.org/10.1016/j.trac.2010.08.009
Bregnhøj, M., Westberg, M., Minaev, B.F., and Ogilby, P.R., Acc. Chem. Res., 2017, vol. 50, no. 8, p. 1920. https://doi.org/10.1021/acs.accounts.7b00169
Khalitova, L.R., Antipin, A.V., Grabovskii, S.A., and Kabal’nova, N.N., High Energy Chem., 2018, vol. 52, no. 5, p. 446.
Vendillo, V.P. and Emel’janov, Yu.M., Zavod. Lab. (USSR), 1959, p. 1401.
Thompson, Q., J. Am. Chem. Soc., 1961, vol. 83, no. 4, p. 845. https://doi.org/10.1021/ja01465a027
Khursan, S.L., Khalizov, A.F., Avzyanova, E.V., Yakupov, M.Z., and Shereshovets, V.V., Russ. J. Phys. Chem., 2001, vol. 75, no. 7, p. 1107.
Koenig, M., Barrau, J., and Hamida, N.B., J. Organomet. Chem., 1988, vol. 356, p. 133.
Shereshovets, V.V., Khursan, S.L., Komissarov, V.D., and Tolstikov, G.A., Usp. Khim., 2001, vol. 70, no. 2, p. 123.
Avzyanova, E.V., Timerghazin, Q.K., Khalizov, A.F., Khursan, S.L., Spirikhin, L.V., and Shereshovets, V.V., J. Phys. Org. Chem., 2000, vol. 13, no. 2, p. 87.
Khursan, S.L., PATAI’s Chemistry of Functional Groups: The Chemistry of Peroxides, Greer, A. and Liebman, J.F., Eds., Chichester: Wiley, 2014, vol. 3, part 2, p. 125.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by S. Zatonsky
Rights and permissions
About this article
Cite this article
Khalitova, L.R., Grabovskii, S.A. & Kabal’nova, N.N. Formation of Singlet Oxygen during Thermal Degradation of Hydrotrioxides of Triorganosilanes. High Energy Chem 53, 435–437 (2019). https://doi.org/10.1134/S0018143919060109
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0018143919060109